Figures & data
Figure 1. Knockdown of DLK increases mouse ES cell relative cell numbers and expression of Nanog protein. D3 mouse ES cells were transfected with sh-Control, sh-DLK-1 and sh-DLK-2 separately, and selected with 1μg/ml puromycin. (A) Relative cell numbers increased in mouse ES cells transfected with sh-DLKs while compared to sh-control. Relative cell numbers were analyzed by the Alamar blue assay. (B) Absolute cell numbers increased in sh-DLK transfectants while compared to sh-control. Absolute cell numbers were measured by the trypan blue exclusion assay. (C) Mouse ES cells transfected with sh-DLKs had more and larger colonies. The alkaline phosphatase activity was stained and photographed under microscope. (D) Both colony numbers and colony sizes increased in sh-DLK ES cells. Colonies stained with alkaline phosphatase on 24-well plates were photographed and calculated. (E) Nanog and Oct4 proteins were upregulated in sh-DLK ES cells. The expression levels of the indicated proteins were detected by Western blotting. The error bars in the figures represent standard error of the mean (mean±SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).
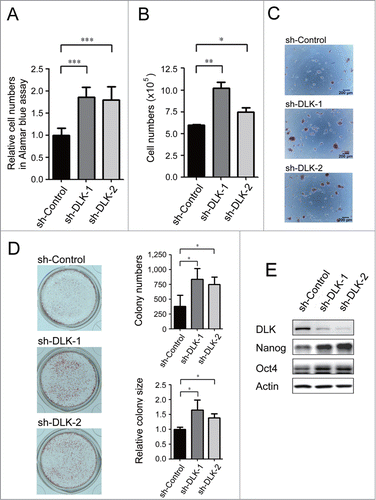
Figure 2. DLK activity is upregulated upon differentiation. (A) The mRNA expression levels of DLK increased upon differentiation. DLK, Nanog, and Oct4 transcripts from D3 mouse ES cells and mouse EBs (6th and 12th days) were quantified by real-time quantitative PCR analyses. Mouse EBs were prepared by suspension method described in the material and methods. (B) The protein expression level of DLK, Nanog, and Oct4 proteins in mouse ES cells and EBs were detected by Western blotting. (C) DLK enzyme activity increased upon differentiation (EB). DLK protein in mouse ES cells and EBs (6th and 12th days) were precipitated with DLK antibody, and aliquoted for DLK kinase activity assays and Western blotting. Myelin Basic Protein (MBP) was used as a DLK substrate to evaluate DLK kinase activity. (D) Nanog protein level decreased upon the removal of LIF. Mouse ES cells maintained in feeder-free conditions were cultured with medium contained 1,000 units/ml of LIF, 500 units/ml of LIF, or without LIF. These cells were harvested for analysis of DLK, Nanog, and Oct4 protein expression by Western blotting. (E) DLK kinase activity increased upon LIF removal or reduction. Mouse ES cells maintained in feeder free conditions were cultured with medium contained 1,000 units/ml of LIF, 500 units/ml of LIF, or without LIF. DLK kinase activity was measured. The error bars in the figures represent standard error of the mean (mean±SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).
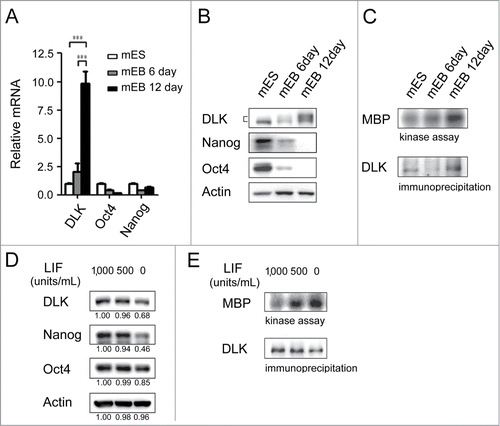
Figure 3. Overexpression of DLK reduces mouse ES cell numbers and the expression of Nanog. (A) The protein expression amounts in DLK overexpression mouse ES cells were similar in Day 15 and Day 20 EBs. DLK protein was detected in DLK overexpressing mouse ES cells (D3) and EBs (5th, 10th, 15th and 20th days) by Western blotting. (B) DLK kinase activity was up-regulated upon the overexpression of DLK. DLK protein precipitated with DLK antibody was aliquoted for DLK kinase activity assays and Western blotting. MBP was used as DLK substrate to evaluate DLK kinase activity. (C) The overexpression of DLK reduced relative cell numbers in ES cells. Relative cell numbers of mouse ES cells transfected with GFP or DLK plasmid were analyzed by the Alamar blue assay in 96-well plates. In the experiment, 2.0 × 104 cells were transfected. (D) Overexpression of DLK slightly down regulated the expression amount of Nanog. The expression of DLK, Nanog and Oct4 proteins were analyzed by Western blotting. The error bars in the figures represent standard error of the mean (mean±SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).
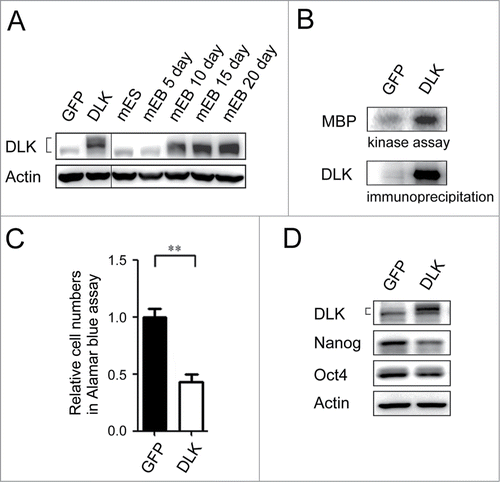
Figure 4. Inhibition of PI3K/Akt signaling increases DLK activity in D3 ES cells. (A) DLK has conserved Akt putative phosphorylation sites in mouse, human, and rat. Mouse, rat and human DLK protein sequences were aligned with CLUSTAL 2.0.12 multiple sequence alignment program.Citation58 Two putative Akt phosphorylation sites in C-terminal of mouse DLK protein are located at Serine 584 and Threonine 659. (B) LY-294002 treatment down-regulated Akt activity and expression of Nanog protein. Western blotting was performed to detect DLK, phospho-Akt at S473, phospho-Akt at T308, total Akt, Nanog and actin after D3 mouse ES cells were treated with 0, 0.25 and 1.0 μM of LY-294002. Under the same condition, (C) DLK activity increased does-dependently upon the treatment of LY-294002. Western blotting and kinase assay using MBP as substrates were carried out as DLK protein was immunoprecipitated. (D) DLK interacted with Akt in the overexpression system. Akt immunoprecipitated products from lysates of mouse ES cells overexpressed DLK, Akt, or DLK plus Akt were hybridized with DLK and Akt antibody in Western blotting analysis. (E) The interaction between endogenous DLK and Akt was demonstrated by co-immunoprecipitation assay. After DLK or Akt protein was immunoprecipitated from D3 mouse ES cells, the co-immunoprecipitated proteins were identified by Western blotting and interaction between endogenous DLK and Akt was demonstrated. The rabbit IgG was used as negative control in the experiment.
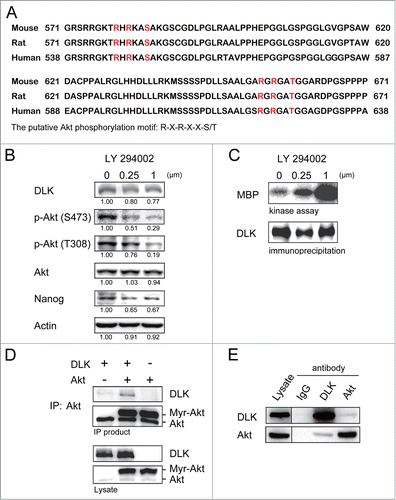
Figure 5. Akt phosphorylates DLK in vitro. (A) Akt phosphorylated both purified His-DLK 541-888, His-DLK 668-888 in vitro. In vitro phosphorylation assay was performed by incubation 400 ng of purified recombinant His-DLK 541-888 and His-DLK 668-888 with or without 15 ng of Akt for 15 minutes. Elutes purified from lysate of E.coli transformed with pGEX-4T vector (modified to expressed 6xHis) were used as negative control. 5 μCi of [γ-32P] ATP was added in each reaction. To quantify these proteins by Western blotting, the [γ-32P]ATP was changed to 20 μM of cold ATP for Western blotting. (B) The mutation of S584A and T659A hampered Akt phosphorylation. In in vitro phosphorylation assay, purified recombinant His-DLK 541-888, His-DLK 541-888 S584A, His-DLK 541-888 T659A, His-DLK 541-888 S584A/T659A or His-DLK 668-888 was incubated with or without of Akt. The experimental conditions were the same as in experiment (A).
![Figure 5. Akt phosphorylates DLK in vitro. (A) Akt phosphorylated both purified His-DLK 541-888, His-DLK 668-888 in vitro. In vitro phosphorylation assay was performed by incubation 400 ng of purified recombinant His-DLK 541-888 and His-DLK 668-888 with or without 15 ng of Akt for 15 minutes. Elutes purified from lysate of E.coli transformed with pGEX-4T vector (modified to expressed 6xHis) were used as negative control. 5 μCi of [γ-32P] ATP was added in each reaction. To quantify these proteins by Western blotting, the [γ-32P]ATP was changed to 20 μM of cold ATP for Western blotting. (B) The mutation of S584A and T659A hampered Akt phosphorylation. In in vitro phosphorylation assay, purified recombinant His-DLK 541-888, His-DLK 541-888 S584A, His-DLK 541-888 T659A, His-DLK 541-888 S584A/T659A or His-DLK 668-888 was incubated with or without of Akt. The experimental conditions were the same as in experiment (A).](/cms/asset/902a1844-110d-4d86-ac7a-7d51299b0c1c/kccy_a_1014144_f0005_b.gif)
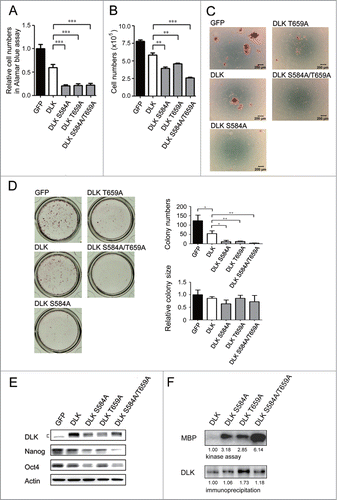
Figure 6. DLK mutated at S584 and T659 significantly reduces mouse ES cell numbers and Nanog expression. D3 mouse ES cells were transfected with GFP, DLK, DLK S584A, DLK T659A, or DLK S584A/T659A. (A) The transfection of DLK S584A, DLK T659A, and DLK S584A/T659A significantly reduced relative cell numbers measured by the Alamar blue assay. (B) Mouse ES cells expressed DLK S584A, DLK T659A, or DLK S584A/T659A displayed significantly reduced cell numbers after counting by trypan blue exclusion assay. (C) Mouse ES cells expressed DLK S584A, DLK T659A, or DLK S584A/T659A dramatically reduced numbers of undifferentiated cell colonies. The alkaline phosphatase activity was stained and photographed under microscope. (D) Colony numbers decreased obviously in mouse ES cells expressed DLK S584A, DLK T659A, or DLK S584A/T659A. Colonies stained with alkaline phosphatase on 24-well were photographed and calculated. (E) Transfection of DLK S584A, DLK T659A, and DLK S584A/T659A DLK downregulated expression amount of Nanog and Oct4. The protein expression levels were detected by Western blotting. (F) DLK mutant at S584A, DLK T659A, or DLK S584A/T659A had higher DLK kinase activity compared to wild type. Wild type DLK and different mutation forms of DLK (DLK S584A, DLK T659A, and DLK S584A/T659A) were expressed, precipitated and aliquoted for Western blotting and kinase assay. In the kinase assay, MBP was used as substrates and the acquired signals were normalized to each precipitated DLKs. The error bars in the figures represent standard error of the mean (mean±SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).