Figures & data
Figure 1. Derivation and characterization of EGFP-HAC. (A) Schematic representation of the 1.1-Mb EGFP-HAC constructed via Cre/loxP-mediated uploading of the CAG-EGFP-polyA cassette with cHS4 insulators onto de novo synthesized alphoidtetO-HAC (see Figure S1 for details and abbreviations) (B) Fluorescent bioimaging of EGFP in living donor CHO cells carrying the assembled EGFP-HAC. (C) Live EGFP imaging in mouse ES cells harboring the EGFP-HAC transferred therein from the CHO cells via the MMCT procedure. Shown are combined transmission light and fluorescent images (B, C). (D) FISH analysis of a metaphase plate of the EGFP-HAC ES cells (clone C1), using a Cy3-labeled DNA probe specific to the alphoid repeat. The DNA was stained with DAPI (blue). The EGFP-HAC is visible as a pink dot (white arrow). (E) Fluorescent immunostaining of the EGFP-HAC ES cells (clone C1) with indicated primary antibodies.
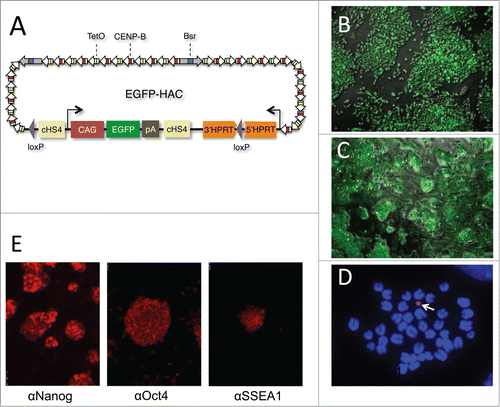
Figure 2. Multilineage differentiation capacity of the EGFP-HAC ES cells within teratomas, exemplified by the formation of ciliated epithelium (ectoderm germ layer), skeletal muscles (mesoderm germ layer), and gut epithelium (endoderm germ layer), all showing EGFP expression following immunofluorescent staining (lower row). H&E – hematoxylin-eosin staining (upper row).
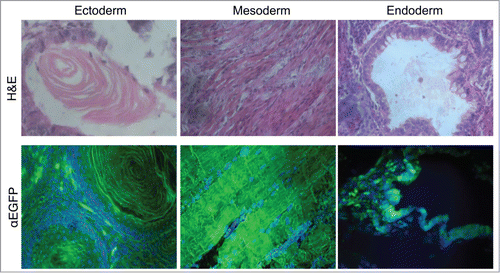
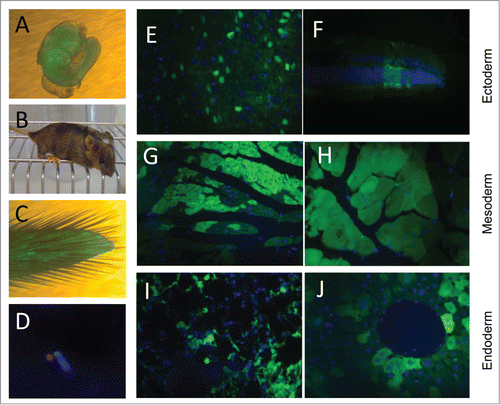
Figure 3. Contribution of EGFP-HAC ES cells to mouse development following blastocyst injection. (A) A representative 10.5 dpc chimeric embryo. (B) A representative 4-week old chimeric mouse. (C) Tail tip of a chimera, which was used to establish primary culture of fibroblasts. (D) FISH analysis of the established fibroblasts, using a Cy3-labeled DNA probe specific to the alphoid repeat. (E–J) EGFP visualization in paraffin sections of representative organs of the chimeric adult mice. Shown are sections of brain (E) and retina (F), both of ectoderm germ layer, heart (G) and skeletal muscles (H) of mesoderm origin, and bronchial epithelial cells (I) and liver hepatocytes (J), both belonging to the endoderm germ layer.