Figures & data
Figure 1. Microtubule regrowth assays in the centrobin-depleted cells. (A) U2OS cells were placed on ice for 45 min and coimmunostained with the α-tubulin (green) and centrobin (red) antibodies. Nuclei were stained with DAPI (blue). The magnified view shows the cytoplasmic centrobin along with stable microtubules. Scale bar, 10 μm. (B) Immunoblot and immunostaining analyses were carried out to confirm centrobin depletion with siRNA transfection. Scale bar, 10 μm. Insets are magnified views of the centrosomes. (C) Microtubule regrowth assays were performed with the centrobin-depleted U2OS cells. The cells were immunostained with the α-tubulin antibody. The cytoplasmic microtubules were enlarged from the 60-second time point. The centrosomal microtubule intensities, the number and length of microtubules from a centrosome, and the number of cells with cytoplasmic microtubules were quantified at the 10-second time point. (D) Microtubule regrowth assays were performed for extended time periods up to 10 min. Microtubule intensities in the centrosome and cytoplasm were determined. Scale bar, 10 μm. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d.. *P < 0.05, in comparison to the control.
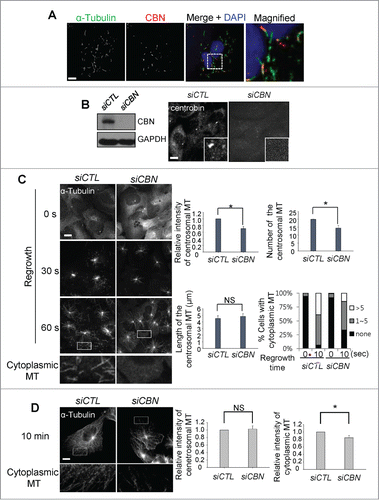
Figure 2. Microtubule regrowth assays with a C-terminus deletion mutant of centrobin. (A) Immunoblot was performed to determine the GFP-CBN and GFP-CBNΔC levels in centrobin-depleted U2OS cells. (B) Microtubule regrowth assays were performed with the centrobin-depleted U2OS cells rescued with GFP-CBN or GFP-CBNΔC. The cells were immunostained with the GFP (green) and α-tubulin (red) antibodies. Scale bar, 10 μm. The centrosomal microtubule intensities, the number of microtubules from a centrosome, and the number of cytoplasmic microtubules were quantified at the 10-second time point. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d.. *P < 0.05, in comparison to the centrobin-depleted cells.
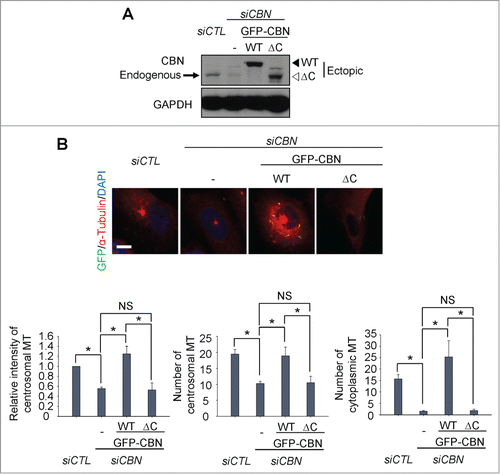
Figure 3. Microtubule regrowth assays with centrobin-PACT. (A) Immunostaining analysis was performed to determine subcellular distribution of GFP-CBN and GFP-CBN- PACT in U2OS cells. The cells were coimmunostained with the GFP (green) and γ-tubulin (red) antibodies. Nuclei were stained with DAPI (blue). Insets are magnified views of the centrosomes. Scale bar, 10 μm. The number of cells with cytoplasmic and/or centrosomal signals of ectopic GFP-CBN was counted. (B) Immunoblot analysis of centrobin was performed with the centrobin-depleted U2OS cells rescued with GFP-CBN and GFP-CBN- PACT. (C) Microtubule regrowth assays were performed with the centrobin-depleted cells rescued with GFP-CBN or GFP-CBN-PACT. The cells were coimmunostained with the GFP (green) and α-tubulin (red) antibodies. Scale bar, 10 μm. The intensity of centrosomal microtubules, the number of microtubules from centrosome, and the number of microtubules at the cytoplasm were quantified at the 10-second time point. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d.. *P < 0.05, in comparison to the centrobin depleted cells. (D) Immunostaining analysis was performed with centrobin-depleted U2OS cells rescued with an excess amount of GFP-CBN-PACT. The magnified view shows the regrown microtubules from the cytoplasmic centrobin. Scale bar, 10 μm.
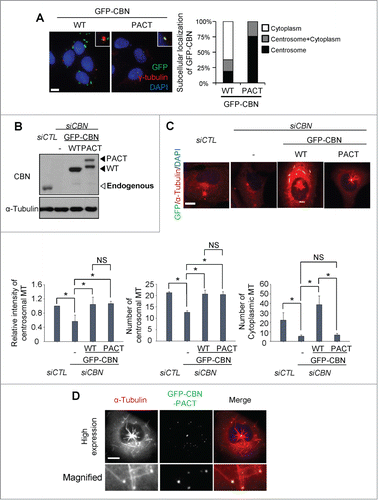
Figure 4. Importance of NEK2 phosphorylation of centrobin in microtubule formation. (A) Immunoblot and immunostaining analyses were carried out to confirm NEK2 depletion with siRNA transfection. Scale bar, 10 μm. (B) Microtubule regrowth assays were performed with the NEK2-depleted U2OS cells. The cells were immunostained with the α-tubulin antibody. The centrosomal microtubule intensities, the number of microtubules from a centrosome, and the number of cells with cytoplasmic microtubules were quantified. Scale bar, 10 μm. (C) Centrobin-depleted U2OS cells were stably rescued with the wild type (WT) or a phospho-resistant mutant of centrobin at T35, S36, S41, S45 (4A). Immunoblot analysis was performed with the centrobin and α-tubulin antibodies. (D) Microtubule regrowth assays were performed for 20 seconds with the centrobin-rescued U2OS cells. The cells were coimmunostained with the GFP (green) and α-tubulin (red) antibodies. Scale bar, 10 μm. The centrosomal microtubule intensities, number of microtubules from a centrosome, and number of cytoplasmic microtubules were quantified at the 10-second time point. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d.. *P < 0.05, in comparison to the centrobin depleted cells.
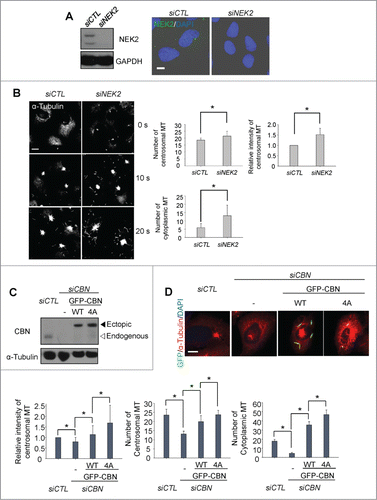
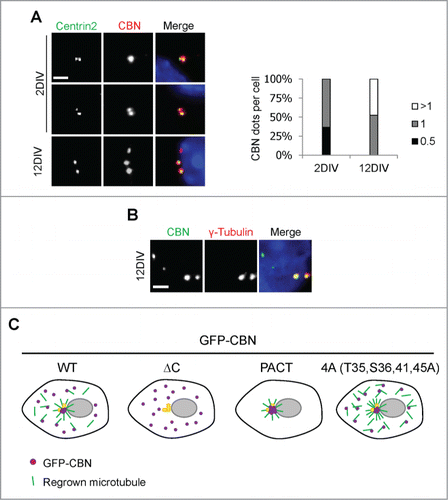
Figure 5. Centrobin expression in mouse hippocampal cells. (A) Mouse hippocampal cells at the 2DIV and 12DIV developmental stages were coimmunostained with the centrobin (red) and centrin2 (green) antibodies. Nuclei were stained with DAPI (blue). Scale bar, 2 μm. The number of centrobin dots in a hippocampal cell was counted. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d. (B) The 12DIV mouse hippocampal cells were coimmunostained with the centrobin (green) and γ-tubulin (red) antibodies. Nuclei were stained with DAPI (blue). Scale bar, 2 μm. (C) Model. Centrobin in both the centrosome and cytoplasm contributes to microtubule nucleation/stabilization. NEK2 phosphorylation reduces the microtubule nucleation/stabilization activity of centrobin.