Figures & data
Figure 1. PTEN deficiency impairs heterochromatin structure. (A) Wild-type (WT) PTEN, PTEN knockout (PKO), or PTEN re-expressed in PTEN knockout (WT/PKO) MEF cells were stained with H3K9me3 (red) and nuclei (DAPI, blue) and western blots (WB) of PTEN protein level is shown below. (B) Immunofluorescent staining revealing H3K9me3 foci in control siRNA (Ctrl) and PTEN siRNA (Psi) knockdown U2OS cells and corresponding WB analysis. (C) RT-qPCR of major and minor DNA satellites in WT PTEN and PKO MEF cells. Ct values of each sample were normalized to GAPDH expression. Error bars indicate s.d. (D) RT-qPCR of Satα and mcBox in control or stable PTEN-knockdown (PTEN sh2) MCF-10A cells. Ct values of each sample were normalized to GAPDH expression. Error bars indicate s.d. (E) ChIP-qPCR analysis in control or stable PTEN-knockdown (Psh2) MCF-10A cells. The occupancy of H3K9me3 at the Satα locus was analyzed by qPCR (2% input). WB analysis of expression levels in stable control and PTEN sh2 cells. (F) MNase digestion assay in MCF-10A with stable (Psh) (left) or U2OS cells with transient (Psi) PTEN knockdown (right).
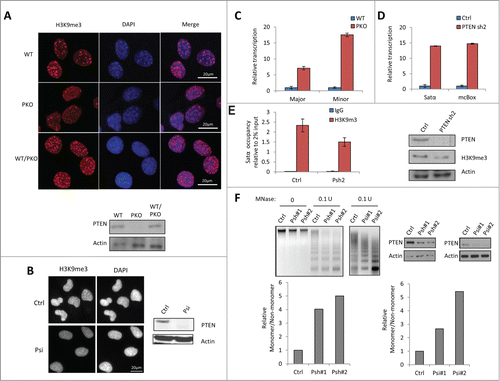
Figure 2. PTEN regulates heterochromatin structure through stabilizing HP1α. (A) GST pull-down assay with WT PTEN or PKO MEF cell lysates, which were incubated with GST or GST-HP1α conjugated beads. The pull-down assay was conducted in duplicate (lanes 2 and 3). (B) Co-IP assay was conducted with U2OS cells transfected with HA (left) or HA-PTEN in MEF cells (right). 4% Input was used. (C) In vitro direct binding assay. Recombinant GST- and GST-HP1α was synthesized via bacteria contructs. PTEN was synthesized by quick couple transcription translation system kit. PTEN and GST-HP1α were incubated and analyzed by WB analysis. (D) MEF cells were transfected with GFP-HP1α and then fractionated by NP-40 and IP was performed in cytosolic (Cyto) and nuclear (Nuc) fractions using anti-HP1α antibody. (E) MEF cells were fractionated by NP-40 and IP was performed using cytosolic (Cyto) and nuclear (Nu) fractions using anti-PTEN antibody. (F) Representative WB of heterochromatin proteins in WT PTEN or PKO MEF cells (left). Quantitative HP1α expression level relative to actin expression from 3 independent experiments (right). Error bars indicate s.d. (G) Immunofluorescent staining revealing of HP1α foci (red) and DNA (blue) in WT and PKO MEF cells. (H) U2OS cells were transfected with control (Ctrl) or PTEN siRNA (Psi). The PTEN-knockdown cells were further treated with PI3K inhibitor, LY294002 (LY) and protein expression was analyzed by WB. (I) Control or PTEN siRNA–transfected U2OS cells were treated with CHX, and analyzed by WB (top). The relative HP1α protein abundance was obtained by measuring the band intensities using ImageJ, and normalizing to actin expression and then to the time point without the addition of CHX (bottom). The half-life of HP1α in WT MEF cells is >24 h and in PKO cells 6 h. (J) MEF and PKO cells were treated with MG132 for 6 h and analyzed by WB. (K) U2OS cells were transfected with control (Ctrl) or PTEN siRNA (Psi), and 24 h later GFP-HP1α and His-ubiquitin (His-Ub) plasmids. Cells were harvested 24 h later and the His-ubiquitin–tagged proteins were purified by Ni-NTA resin. The ubiquitinated HP1α was detected with an anti-GFP antibody. (L) RT-qPCR was performed to determine the Satα level (right). Ct values of each sample were normalized to GAPDH. Error bars indicate s.d. Western blot analysis of targeted genes.
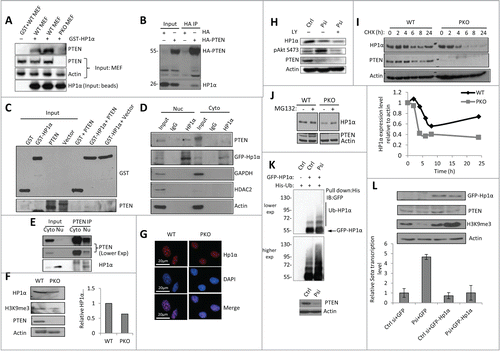
Figure 3. C-terminus is required for PTEN to Stabilize HP1α. (A) Knockdown-mutant rescue assay in U2OS cells that were transfected with control (Ctrl) or 2 sets of PTEN siRNA (Psi#1 and Psi#2). After 24 h, empty vector, WT PTEN, or the phosphatase dead PTEN mutant, C124S, were overexpressed (top). The cells were harvested 24 h after the second transfection, and analyzed by RT-qPCR and WB (top). PTEN-knockdown U2OS cells were rescued with empty vector, WT PTEN or various cancer-associated PTEN mutants (bottom). Ct values of each sample were normalized to GAPDH expression. Error bars indicate s.d. WB is shown above the corresponding RT-qPCR condition in order to display exogenous protein expression level. (B) Protein stability of HP1α determined by WB in U2OS cells that were transfected with HA-WT PTEN or HA-PTEN Y336*, and treated with CHX for up to 27 h after transfection. The band intensities were measured by ImageJ and the relative HP1α protein level was normalized to actin expression (bottom). The half-life of HP1α in PTEN Y336* is 5 h compared to 24 h in PTEN WT cells. (C) His-ubiquitin and GFP-HP1α were co-transfected with WT, vector or PTEN Y336*. Cells were harvested 48 h after transfection and the His-ubiquitin–tagged proteins were purified by Ni-NTA resin. The ubiquitinated HP1α was detected with an anti-GFP antibody. (D) U2OS cells were transfected with HA-WT PTEN (WT) or HA-PTEN Y336* (336*), fractionated to obtain cytosolic (cyto) and nuclear (nuc) proteins and analyzed by WB. (E) Flag-PTEN WT, HA-vector, or HA-PTEN Y336* was transfected into U2OS cells, fractionated, and whole cell lysate, cytosolic, and nuclear protein extracts were then analyzed by WB. (F) Co-IP was conducted to determine the interaction between WT PTEN and HP1α in the presence of PTEN Y336*. BT549 cells expressing WT PTEN (BT549 PTEN), Vet (BT549 Vet), or PTEN Y336* (PT549 336*) were transfected with HA-PTEN WT, HA-vet, or HA-PTEN Y336*. A co-IP was performed with anti-HA antibody and probed for PTEN and HP1α.
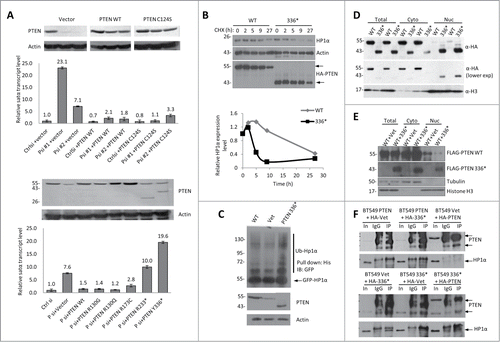
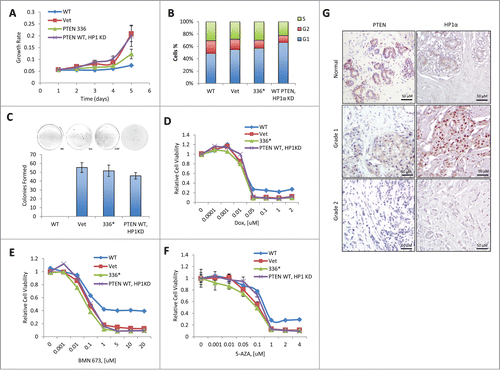
Figure 4. The heterochromatic function of PTEN is required for its tumor-suppressive activity. (A) BT-549 cells were reconstituted with empty vector (Vet), WT PTEN (WT), or PTEN Y336* (336*) or PTEN WT HP1α KD. Relative cell growth rates were determined by MTT assay. The results were presented as mean of 3 independent experiments. Error bars indicate s.d. (B) BT-549 cells reconstituted with Vet, WT, 336*, or PTEN WT HP1α KD were analyzed for the cell cycle distribution by flow cytometry. (C) BT-549 cells reconstituted with Vet, WT, 336* or PTEN WT HP1αKD anchorage-independent cell growth determined by soft agar assays. The results were presented as mean of 3 independent experiments. Error bars indicate s.d. (D) BT-549 cells reconstituted with Vet, WT, 336* or PTEN WT HP1αKD were treated with doxorubicin (Dox). Relative cell viability was determined by MTT assay. The results shown are a mean of 3 independent experiments. Error bars indicate s.d. (E) BT-549 cells reconstituted with Vet, WT, 336* or PTEN WT HP1αKD were treated with BMN673. Relative cell viability was determined by MTT assay. The results were presented as mean of 3 independent experiments. Error bars indicate s.d. (F) BT-549 cells reconstituted with Vet, WT, 336* or PTEN WT HP1αKD were treated with 5-aza-2′-deoxycytidine (5AZA). Relative cell viability was determined by MTT assay. The results were presented as mean of 3 independent experiments. Error bars indicate s.d. (G) Representative immunohistochemistry tissue array staining for HP1α and PTEN expression in 6 individual breast carcinoma patients. Grade 1 = well differentiated. Cells appear normal and are not growing rapidly. Grade 2 = moderately-differentiated. Cells appear slightly different than normal.