Figures & data
Figure 1. Cdk1 regulates Plk1 localization and activation. (A) Plk1 primary structure and organization. Plk1 contains a N-terminal serine/threonine kinase domain (blue) and a C-terminal Polo-binding domain (PBD) containing 2 Polo Boxes (PB1 and PB2 in green) separated by a loop (L2, red). The Inter-Domain linker (IDL, orange) links the PBD and the KD. The activating T-loop phosphorylation site (T210) located in the KD is shown with a red line. (B) Mechanisms of Plk1 regulation. In the auto-inhibited Plk1 state (a), the PBD engages in an intramolecular interaction with the KD via the loop region L2, which inhibits the KD, and the IDL prevents access of the activating site in the T-loop to Aurora A kinases. Phospho-peptide binding (b) induces a conformational change of the L2 region that disrupts the KD-PBD interaction and contributes to Plk1 activation. Phosphorylation of the T-loop releases the inhibitory effect of the IDL (c). These two mechanisms contribute to Plk1 activation (d). CDK-1-Cyclin B (yellow) primes several substrates for interaction with the PBD thereby contributing to its basal activation and phosphorylates SPAT-1 to promote its interaction with PLK-1, possibly contributing to displace the IDL, which facilitates T-loop phosphorylation.
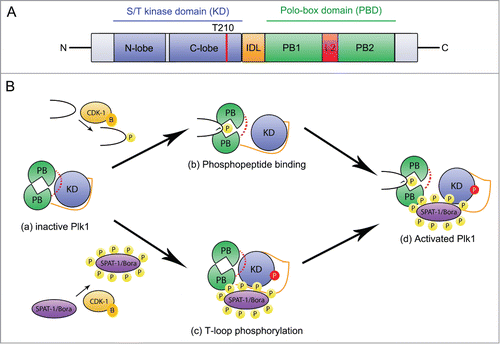
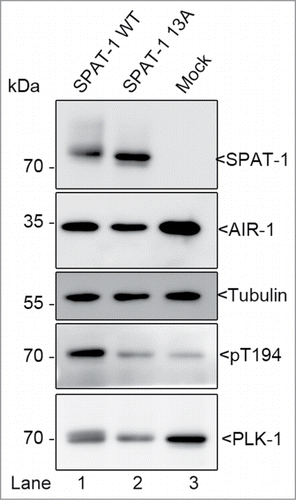
Figure 2. SPAT-1 wild-type but not the non-phoshorylatable SPAT-1 13A stimulates PLK-1 T-loop phosphorylation by AIR-1. Westernblot analysis of protein extracts obtained from baculovirus-infected Sf9 cells co-expressing AIR-1, PLK-1, and SPAT-1 WT (Lane 1) or SPAT-1 13A (lane 2) or co-expressing only AIR-1 and PLK-1 (Lane 3) revealed with 6xHis (His-AIR-1), SPAT-1, PLK-1, Tubulin and the T-loop phosphospecific antibody (T194 in PLK-1).