Figures & data
Figure 1. ER stress-associated apoptosis was involved in H/R-induced endothelial injury. (A-C) Cell lysates from HUVECs were immunoblotted with antibodies of GRP78, caspase-12 and CHOP with different reoxygenation time course. (D-F) Protein expression of GRP78, caspase-12 and CHOP in endothelial cells treated with an ER stress inhibitor 4-PBA (10−4 M) in the context of H/R. GAPDH served as internal control. Open bar, normoxia; filled bar, H/R. The data expressed as mean ± SEM in each bar graph represent the average of 4 independent experiments. *P < 0.05 and **P < 0.01 vs Con; ##P < 0.01 vs H/R.
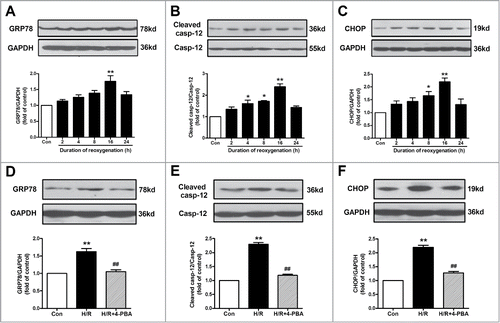
Figure 2. ACh diminished H/R-induced ER stress and apoptosis in endothelial cells in a dose-dependent manner. (A) Representative immunoblots for GRP78, caspase-12, CHOP and GAPDH. (B–D) Quantitative analysis results of the expression of GRP78, caspase-12 and CHOP after ACh treatment in the context of H/R. Open bar, normoxia; filled bar, H/R. The data expressed as mean ± SEM in each bar graph represent the average of 4 independent experiments. **P <0.01 vs Con; #P < 0.05 and ##P < 0.01 vs H/R.
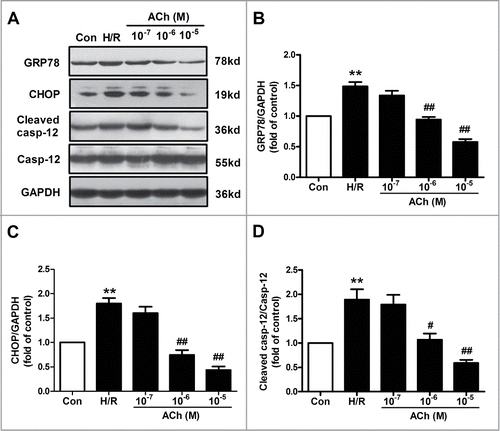
Figure 3. ACh inhibited ER-related apoptosis through M3 AChR. (A) Representative immunoblots for GRP78, caspase-12 and CHOP. (B) ACh treatment decreased the upregulated expression of GRP78, cleaved caspase-12 and CHOP induced by H/R. The beneficial effects of ACh was abolished by M3 AChR antagonist 4-DAMP (10−6 M). (C and D) ACh inhibited CHOP immunofluorescence during H/R. Scale bar = 25 μm. The data expressed as mean ± SEM in each bar graph represent the average of 4 independent experiments. **P < 0.01 vs Con; ##P < 0.01 vs H/R; &P < 0.05 and &&P < 0.01 vs ACh-treated H/R group.
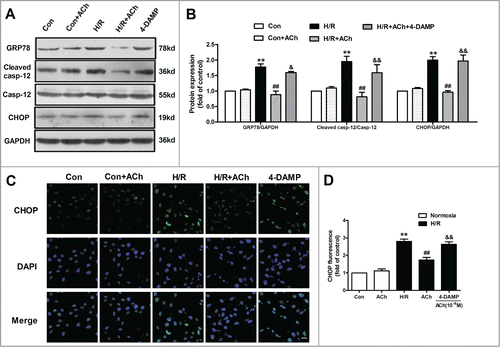
Figure 4. ACh protected against H/R-induced destruction of ER ultrastructure. The transmission electron microscopic observations indicated that ACh could preserve cellular ultrastructural changes triggered by H/R in HUVECs, especially the changes of ER. (A) 20000×, Scale bar = 1 μm. (B) 40000×, Scale bar = 500 nm.
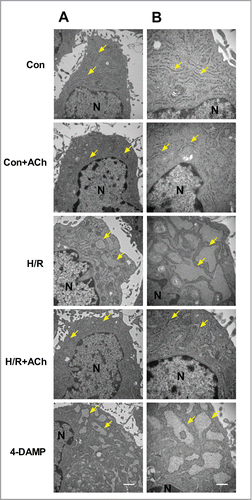
Figure 5. ACh inhibited PERK and IRE1 signaling pathways to protect HUVECs against H/R injury. (A and B) H/R-induced increase in TUNEL-positive cells was inhibited by ACh, 4-DAMP (10−6 M) abrogated the benefits of ACh in the context of H/R. Scale bar = 200 μm. The changes of p-IRE1/IRE1 (C), p-PERK/PERK (D) and ATF6 (E) expression were examined by Western blot. Open bar, normoxia; filled bar, H/R. The data expressed as mean ± SEM in each bar graph represent the average of 4 independent experiments. **P < 0.01 vs Con; ##P < 0.01 vs H/R; &P < 0.05 and &&P < 0.01 vs ACh-treated H/R group.
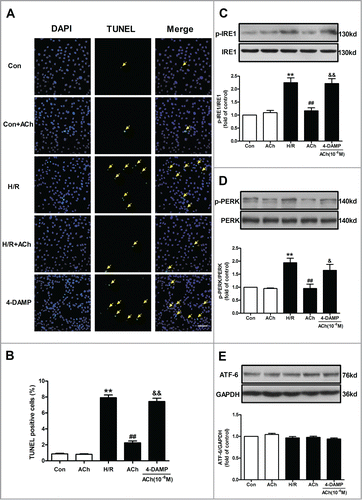
Figure 6. Knockdown of M3 AChR blocked the effects of ACh during H/R. (A) The silencing efficiency of M3 AChR siRNA. Cells were transfected with siRNA followed by H/R. The expression changes of p-IRE1/IRE1 (B) and p-PERK/PERK (C) were determined after M3 AChR/NC siRNA with or without ACh. (D and E) Representative immunoblots and quantitative analysis of GRP78, cleaved caspase-12 and CHOP after M3 AChR/NC siRNA with or without ACh. The data expressed as mean ± SEM in each bar graph represent the average of 4 independent experiments. *P < 0.01 vs NC siRNA group. #P < 0.01 vs ACh-treated NC siRNA group.
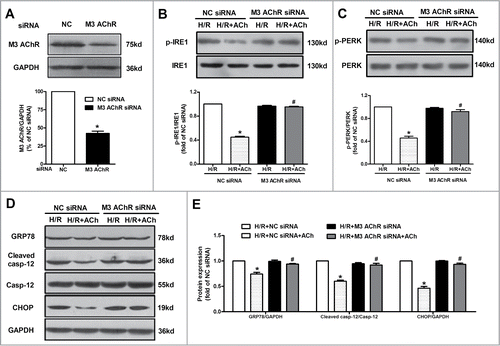
Figure 7. AMPK activation was responsible for the protection of ACh in the context of H/R. (A) ACh increased the phosphorylation of AMPK after H/R, which was blocked by 4-DAMP (10−6 M). *P < 0.01 vs Con; #P < 0.01 vs H/R; &P < 0.01 vs ACh-treated H/R group. (B) M3 AChR-depleted cells reduced the AMPK phosphorylation in the context of H/R. (C) The silencing efficiency of AMPK siRNA. (D) Representative immunoblots and quantitative analysis of GRP78, cleaved caspase-12, CHOP, p-PERK, PERK, p-IRE1 and IRE1 after AMPK/NC siRNA with or without ACh in the context of H/R. The data expressed as mean ± SEM in each bar graph represent the average of 4 independent experiments. *P < 0.01 vs NC siRNA group. #P < 0.01 vs ACh-treated NC siRNA group.
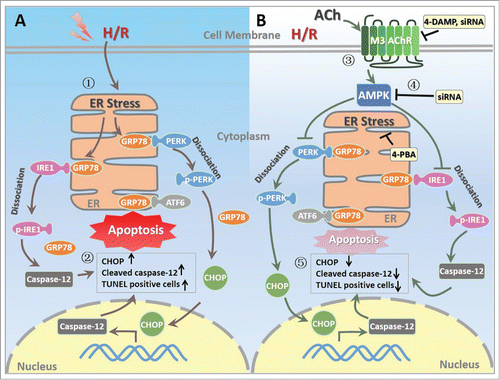
Figure 8. Proposed schematic illustration of the mechanism by which ACh exerts its endothelial protective effects against H/R injury in the present study. (1) H/R induces ER stress in endothelial cell; (2) H/R upregulates the expression of CHOP and cleaved caspase-12 as well as TUNEL positive HUVECs; (3) ACh activates M3 AChR to elicit its endothelial protection; (4) ACh enhances AMPK phosphorylation, thereby inhibits pro-apoptotic cascades; (5) ACh decreases ER stress and apoptosis during H/R in endothelial cells, presumably by M3 AChR signaling pathway. ACh, acetylcholine; AMPK, AMP-activated protein kinase; ATF6, activating transcription factor 6; CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; GRP78, glucose-regulated protein 78; H/R, hypoxia/reoxygenation; IRE1, inositol-requiring kinase 1; M3 AChR, type-3 muscarinic acetylcholine receptor; PERK, protein kinase-like ER kinase; TUNEL, terminal deoxynucleotidyl transferase mediated dUTP-biotin nick end labeling; 4-DAMP, 4-diphenylacetoxy-N-methylpiperidine methiodide; 4-PBA, 4-phenyl butyric acid.