Figures & data
Figure 1. Senescent glioblastoma cells have aberrant centrosomes. (A) Representative images of untreated normal and senescent cells. Centrosomes stained by immunofluorescent staining for γ-tubulin (1:800, white) and nuclei stained with DAPI (blue), inset is a magnification of the centrosomes. To the right is the brightfield grayscale image of the cells showing the presence or absence of SAβGal stain. Normal (non-senescent) cells are characterized by a single nucleus of average size, a typical cytoplasmic size and are negative for SAβGal stain. Senescent cells are characterized by a single enlarged nucleus, increased cytoplasmic size and are positive for SAβGal stain. Scale bar = 10 μm. (B) A histogram showing the centrosome number in normal and senescent U87MG cells. (C) Percentage of normal and senescent cells with aberrant centrosomes in 3 glioblastoma cell lines (U87MG, A172, DBTRG). Centrosomes were classified as aberrant if either supernumerary or separated in an interphase cell. For B and C, a minimum of 50 normal and senescent cells were counted following immunofluorescence staining for γ-tubulin. Data for senescent cells are pooled data from the analysis of 3 separate slides. Statistical significance was determined using Pearson's chi-squared test. * p < 0.05.
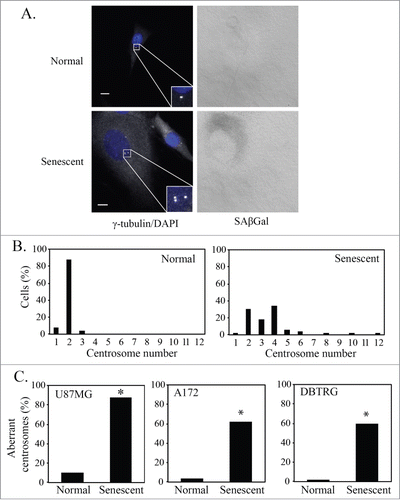
Figure 2. p21-induced senescent glioblastoma cells have aberrant centrosomes. (A) Representative images of p21-induced senescent U87MG and A172 glioblastoma cells after overexpression of p21. Cells underwent immunofluorescence staining for γ-tubulin (white). Nuclei were stained with DAPI (blue) and a brightfield grayscale image of SAβGal stain is included to the right. Cells were transduced with a p21 lentivirus overnight, washed and fixed for staining 4 days later (5 days after initial transduction). Inset is a magnification of the centrosomes. Scale bar = 10 μm. (B) Quantification of normal and senescent (same criteria used in ) glioblastoma cells (U87MG, A172) with aberrant centrosomes after overexpression of p21. Statistical significance was determined using Pearson's chi-square test. * p < 0.05.
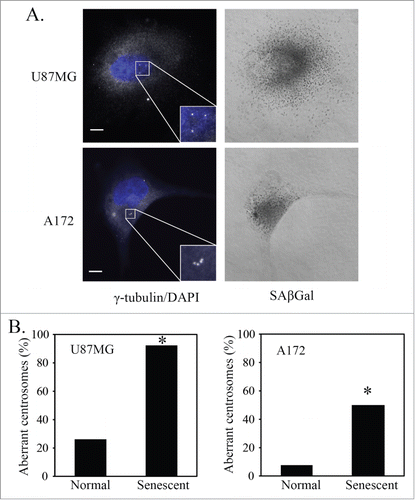
Figure 3. Senescent U87MG cells are polyploid. (A) Chromogenic in situ hybridization (CISH) using a probe for the centromere of chromosome 7. Representative images of normal and senescent (by morphology) U87MG cells. Scale bar = 10 μm. (B) Quantification of CISH staining in U87MG cells (n = 30). The distribution of CISH copy number between normal and senescent cells was significantly different as determined using Pearson's chi-squared test.
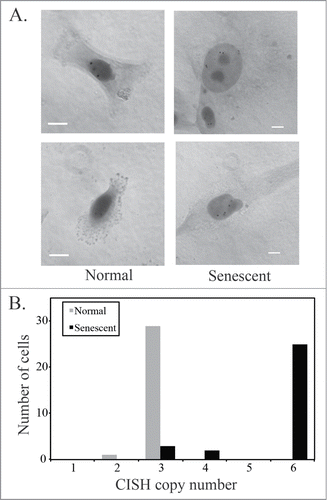
Figure 4. Cyclin expression in senescent glioblastoma cells. (A) Representative images of untreated normal and senescent (by morphology) U87MG cells. Immunofluorescence staining of Cyclin B1 (red) and nuclei are stained with DAPI (blue). Senescent cell of interest is shown using an arrow and the normal cell undergoing metaphase is shown using an asterisk. Scale bar = 10 μm. (B) Immunofluorescence staining of Cyclin E (red) and Ki-67 (green) in an untreated U87MG normal (asterisk) and senescent (arrow) cell. Nuclei are stained with DAPI (blue) and a brightfield grayscale image of SAβGal is on the bottom right. Scale bar = 10 μm.
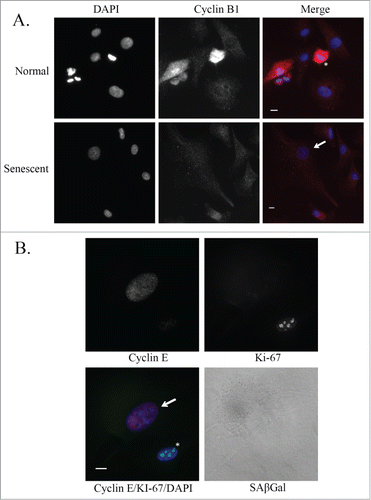
Figure 5. PKCι depletion-induced senescence shows markers of mitotic slippage. U87MG cells were either mock transfected, transfected with control siRNA (control RNA), or siRNA targeting PKCι (iota RNA A and iota RNA E). After 48hr of siRNA, cells were washed and grown for another 3 days in regular media, followed by fixation and staining. (A) Quantification of the percent of SAβGal positive staining of U87MG cells in the 2 experiments used for counting. A minimum of 100 cells over 3 fields of view were counted in each of the 2 separate experiments. (B) Quantification of senescent (same criteria as ) U87MG cells showing aberrant or normal centrosome morphology. Minimum of 30 cells counted for each condition. (C) Quantification of senescent U87MG cells that are positive or negative for Cyclin E. Minimum of 30 cells counted for each condition. (D) Quantification of senescent U87MG cells that are positive or negative for Ki-67. Minimum of 40 cells counted for each condition. (E) CISH staining using a probe for the centromere of chromosome 7 of U87MG cells 5 days after siRNA targeting PKCι. Minimum of 100 cells counted for each condition in 3 separate experiments. Statistical significance was determined using a 2-tailed t-test. Error bars indicate the standard error of the mean. (* p < 0.05)
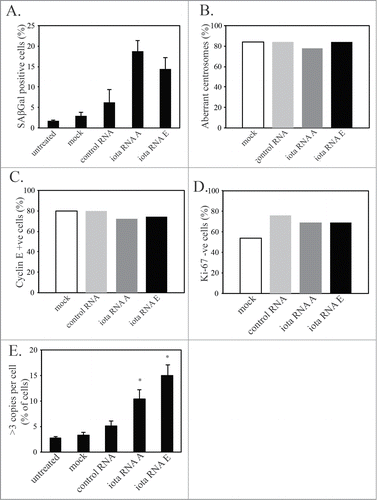
Figure 6. Markers of mitotic slippage in senescent U87MG cells after PKCι depletion. Representative immunofluorescence images of senescent U87MG cells 5 days after siRNA targeting PKCι (iota RNA A). (A) Top left, phase contrast; top right, SAβGal staining (grayscale); bottom left, DAPI; bottom right, γ-tubulin staining centrosomes. Inset is a magnification of the centrosomes. Scale bar = 10 μm. (B) Top left, phase contrast; top right, SAβGal staining (grayscale); bottom left, DAPI; bottom right, Ki-67. Scale bar = 10 μm.
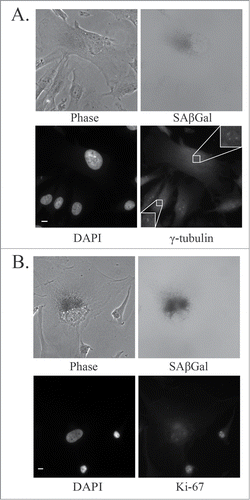
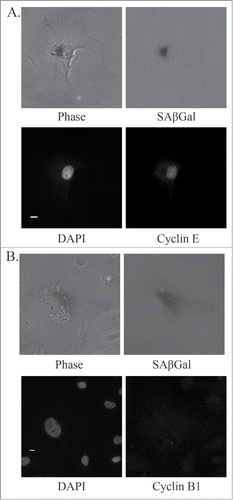
Figure 7. Markers of mitotic slippage in senescent U87MG cells after PKCι depletion. Representative immunofluorescence images of senescent U87MG cells 5 days after siRNA targeting PKCι (iota RNA A). (A) Top left, phase contrast; top right, SAβGal staining (grayscale); bottom left, DAPI; bottom right, Cyclin E. Scale bar = 10 μm. (B) Top left, phase contrast; top right, SAβGal staining (grayscale); bottom left, DAPI; bottom right, Cyclin B1. Scale bar = 10 μm.