Figures & data
Figure 1. Generation and characterization of TMZ-resistant GBM cells. (A) Flowchart for the generation of TMZ-resistant and wash out GBM cells. (B) Induction of apoptosis by TMZ in the A172 cell line. Apoptosis induced by TMZ cells was determined by annexin V staining. No, or negligible apoptosis was detected after 24 hrs (data not shown) whereas apoptosis was detectable at 48 and 72 hrs. This panel reports the results after 72 hrs. (C) Cell cycle analysis in A172 WT and A172 TMZ R cells. Cells were seeded in 6-well culture plates at a density of 5 × 105 cells/well and grown for 48 hrs in the presence of 200 µM TMZ or without drug (A172 WT only). The result of this analysis shows that 66.3% of the WT and 40.3 % of the TMZ R cells accumulate at G2/M upon treatment with TMZ. This indicates that TMZ R cells are a slow-cycling population that can eventually escape the G2 checkpoint. (D) Drug holiday partially restores TMZ sensitivity in GBM TMZ-R cells. GBM3 and GBM5 TMZ-resistant cells were grown in drug-free medium for at least 30 days (GBM3 and GBM5 WO) and then challenged with TMZ. Cell viability was measured by MTT assay 72 hrs. after treatment. The significance of the difference between WT, TMZR and WO cells is reported in Table S1E: Reversibility of apoptosis resistance in the TMZR GBM 3A cells after TMZ challenge, measured by annexin V staining. GBM 3 TMZR (Panel A) and GBM 3A TMZR were obtained from the same parental culture (GBM3) in 2 biologically distinct experiments of induction of drug resistance with different TMZ concentrations (400 µM for GBM 3 and 200 µM for GBM 3A). Apoptosis was measured after 72 hrs. The observed differences were significant at P < 0.05 (*) or P < 0.01 (**) (Bonferroni post-hoc).
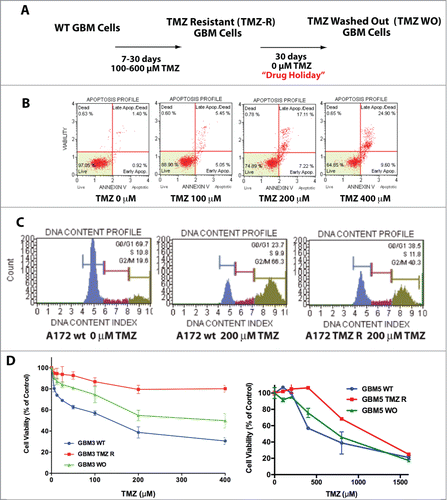
Figure 2. KDM genes expression in GBM CSC cells and tumors. (A) Expression of KDM genes in 2 TMZ-resistant GBM CSC cells analyzed by qPCR in WT GBM3, GBM5 and in their TMZ-R and WO derived cultures. Fold change is relative to the expression of the WT parental cells. (B) Comparison of the mean expression levels of KDM4A, 4B, 5A and 5B in GBM and normal brain. (C) Comparison of the mean expression levels of KDM1A and KDM5A in primary GBM, recurrent GBM and normal brain. In Panels B and C the box represents the 10–90 percentile and whiskers the min-max level of expression. Significance of the mean differences was evaluated by t-test and ANOVA.
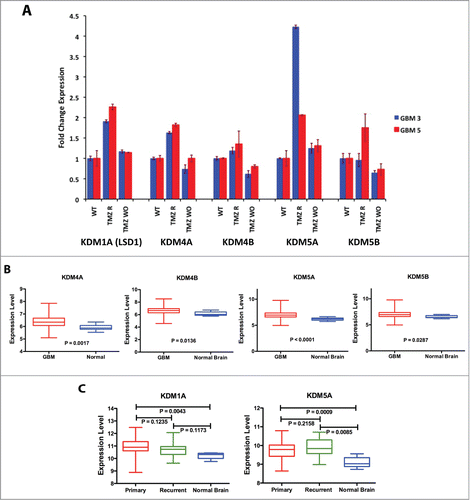
Figure 3. KDM5A is one of the determinants for TMZ resistance in GBM cells. (A) Cell viability measured by MTT assay in mock and KDM5A transfected A172 cells 48 hrs. after TMZ treatment (IC50 A172 WT: 243 µM; IC50 A172 KDM5A: 810 µM) . The observed differences were significant at P < 0.01 (**) or P < 0.001 (***) (2-way ANOVA and Bonferroni post-hoc). (B) Cell viability measured by MTT assay in mock and KDM5A transfected GBM3 cells 48 hrs. after TMZ treatment. IC50 for GBM3 WT and KDM5A were 183 and 641 µM, respectively. The higher IC50 value for GBM3 WT reported in this panel compared to Panel D of reflects the different incubation times in the 2 experiments (72 and 48 hrs). The observed difference were significant at P < 0.01 (**) or P < 0.001 (***) (2-way ANOVA and Bonferroni post-hoc). (C) Protection from apoptosis induced by TMZ by exogenous KDM5A. A172 cells mock or transiently transfected with KDM5A were treated with TMZ at different concentration and the level of apoptosis was measured after 24 hrs. by annexin V staining. The different sensitivity to apoptosis induced by TMZ in KDM5A and mock-transfected cells was highly significant (P < 0.0001) by linear regression analysis. (D) Silencing of the KDM5A gene sensitizes GBM cells to TMZ. Apoptosis is significantly induced by TMZ in GBM 3 and A172 TMZ-R derivatives transfected with shRNA-KDM5A and with the shGFP sequence. The cells were treated with TMZ 48 hrs. after transfection and apoptosis was measured by Annexin V staining 24 hrs after treatment. P < 0.05 (*) or P < 0.001 (***).
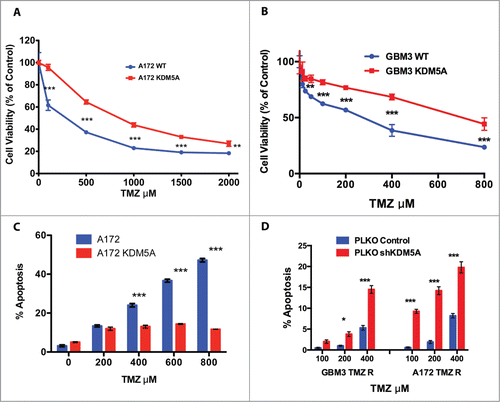
Figure 4. Induction of TMZ resistance, MGMT expression and ABC-transporters functionality. (A) Expression of MGMT in WT GBM3, GBM5 and A172, utilizing the SK N AS neuroblastoma cell line as reference positive expression control. (B) Induction of TMZ resistance in GBM3 cells results in the transient expression of MGMT. After WO the expression returns to baseline levels. The exogenous expression of KDM5A does not change MGMT expression. GBM3 WT cells were utilized as reference positive control. (C) Induction of TMZ resistance does not increase MGMT expression in GBM5 cells. GBM5 WT cells were utilized as reference positive control. (D) Comparison of the mean expression levels of KDM1A and KDM5A in a 483 primary GBM dataset from TCGA. The box represents the 10–90 percentile and whiskers the min-max level of expression. Normalized mean expression values were: −0.964 for MGMT and 0.296 for KDM5A. (E) Relative mRNA expression levels of ABCB1, ABCC1 and ABCG2 in A172 WT and TMZ R cells as determined by qPCR. Fold change is relative to the expression of TMZ WT cells. (F) Cytotoxic effect of doxorubicin on A172 WT and TMZ-R cells. Cells were treated with doxorubicin (0–1 μM) for 48h and viability was determined by MTT assays. (G) Immunofluorescence images of subcellular doxorubicin distribution in A172 WT and TMZ R. Cells were treated with autofluorescent DOX (red) for 24h, nuclei and cell membranes were counterstained with DAPI (blue) and DiO (green), respectively. Overlay of images shows the predominant nuclear localization (pink) of DOX in most cells of both A172 cultures (original magnification 40X).
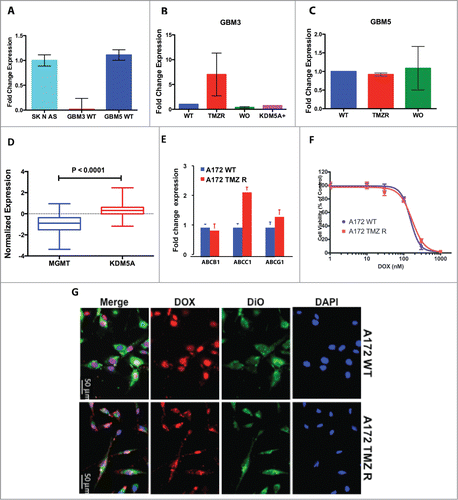
Figure 5. Effect of HDAC inhibitors on TMZ-R cells. (A) Resistance to apoptosis induced by TMZ in TMZ-resistant GBM cells and sensitivity to the HDAC-inhibitor SAHA. TMZ-R A172 cells were treated with 200 µM TMZ or with 1 µM SAHA or their combination (200 µM TMZ and 1 µM SAHA); apoptosis was measured by Annexin V staining after 72 hrs. (B) The combination of SAHA and TMZ exerts a significantly stronger effect compared to that of the each molecule utilized as single agent. Digital images of the wells (C) were collected and the colonies (at least 10 cells) were manually counted. (C) Colony growth of A172 WT (blue) and A172 TMZ R cells (red) treated for 24 or 72 hrs with SAHA. Identical results were obtained plating 2,500 or 5,000 cells. The result presented in this panel is that obtained with 2,500 cells/well. (D) Synergistic effects induced by TMZ (1–4 µM) and SAHA (62.5–1000 µM) combined treatment of A172 TMZ-R and GBM 3 cells evaluated by isobologram analysis according to the Chou-Talalay algorithm utilizing the CompuSyn software (www.combosyn.com). (E) Colony growth of A172 WT and A172 TMZ R treated with SAHA. A172 TMZ R (Panel A) and A172 WT (B) cells were treated for 24 or 72 hrs with serial dilutions of SAHA (From 10 to 1.25 µM), at the end of the treatment the cells were seeded in a 6 well plate at a density of 2,500 cells/well in tissue culture medium without TMZ and were grown for further 10 days in medium without drugs. At the end of the incubation period, the cells were fixed with methanol and stained with Crystal Violet.
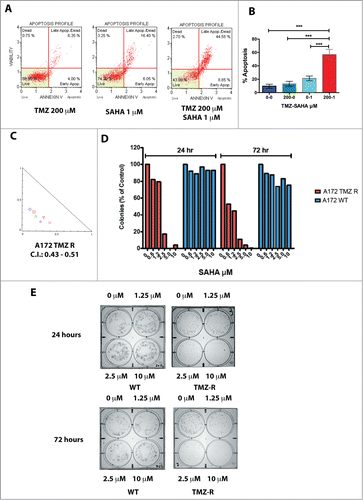
Figure 6. Relation between stemness and TMZ sensitivity. (A) Sensitivity to TMZ of GBM CSCs grown under stem or differentiated conditions. The P values were determined by ANOVA with the Tukey's test and are indicated for each significant point (*: P < 0.05; **: P < 0.01; *** P < 0.001 vs differentiated cells). (B) mRNA expression levels of ABCB1, ABCC1, ABCG2 and KDM5A in GBM3 differentiated cells relative to the same cells grown under stem-permissive conditions determined by quantitative PCR.
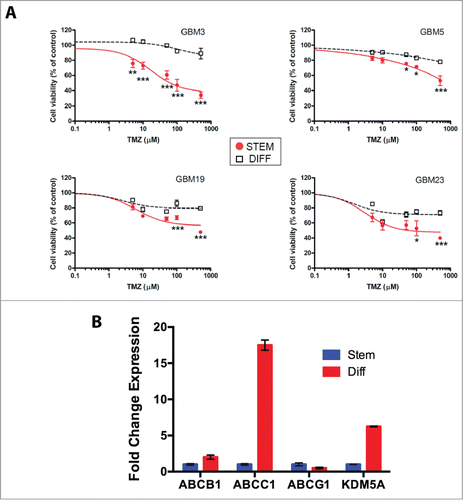
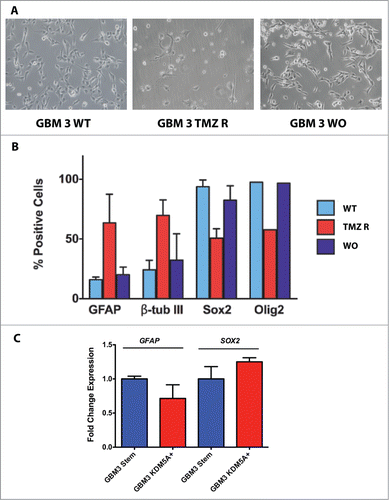
Figure 7. Stem and differentiation markers in GBM3 WT cells and in TMZ-R and WO cultures. (A) Bright field images of GBM 3 WT culture before treatment, and of TMZ R and WO cultures. (B) Expression of stem and differentiation markers evaluated by immunofluorescence in WT, TMZ R and WO conditions. Results are expressed as % mean of marker-positive cells from 3 independent experiments and bar represents standard deviations of mean. (C) Real time PCR analysis of GFAP and SOX2 expression in GBM3 WT cells and in GBM3 cells transfected with the pcDNA3/HA-FLAG-RBP2 construct.