Figures & data
Figure 1. BCL10 is recruited to sites of DNA damage. (A) Immunodetection of BCL10 and γ-H2AX in T47D cells transfected with control siRNA (CTL siRNA) or BCL10 siRNA (BCL10 siRNA). Quantification of the average number of cryptic γ-H2AX foci per cells is shown on the right. Efficiency of BCL10 knockdown was shown by an immunoblot on the top. (B) Immunodetection of BCL10 and γ-H2AX 60 min after IR (2 Gy) irradiation. The right panel shows Pearson's correlation coefficient values for co-localization of γ-H2AX and BCL10 with and without IR.
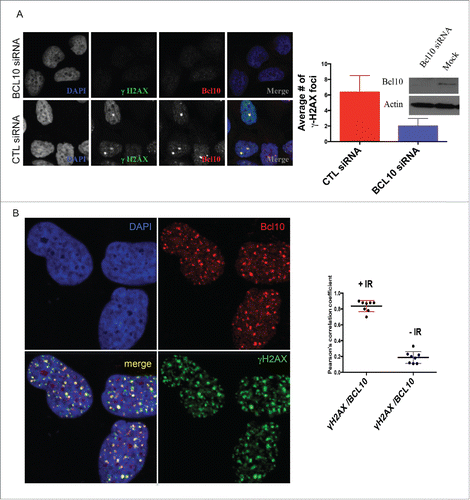
Figure 2. Kinetics of DNA damage-induced BCL10 foci formation. Immunodetection of BCL10 and γ-H2AX or p-ATM (S1981) at the indicated time points after IR (2 Gy) irradiation. Quantification of the average number of IR-induced BCL10/γ-H2AX or BCL10/p-ATM foci per cells is shown on the bottom. (A) BCL10/γ-H2AX. (B) BCL10/p-ATM.
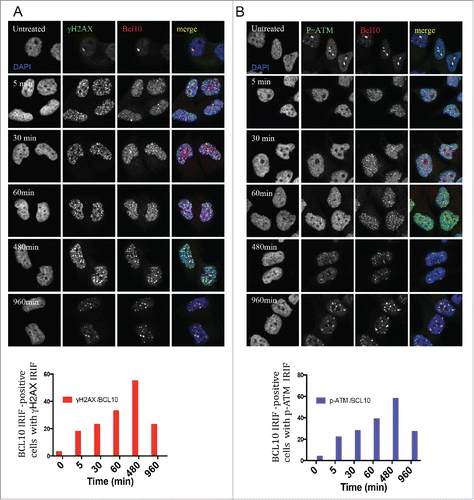
Figure 3. MALT1 is dispensable for γ-H2AX cryptic foci formation. (A) Immunodetection of BCL10 and γ-H2AX in T47D cells transfected with control siRNA (CTL siRNA), or MALT1 siRNA (MALT1 siRNA). Quantification of the average number of cryptic γ-H2AX foci per cells is shown on the right. MALT1 knocking down efficiency was shown by an immunoblot on the top. (B) The effect of BCL10 knock down on NFκB activation in response to DNA damage. NFκB activation was measured by the translocation of p65 into the nucleus in response to DNA damage with or without BCL10 depletion. Quantification of the p65 nuclear translocation was measured.
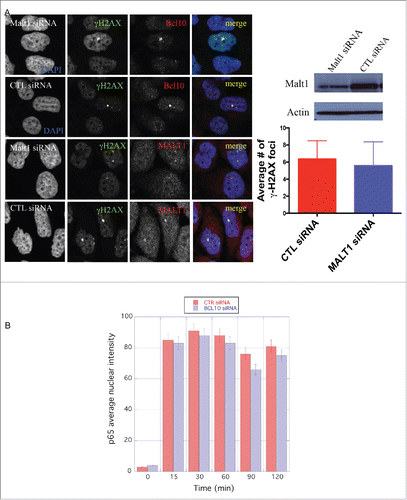
Figure 4. H2AX-dependent but ATM-independent recruitment of BCL10 to the DNA damage sites. (A) Immunodetection of BCL10 and MRE11 in T47D cells transfected with control siRNA (CTL siRNA) or H2AX siRNA (H2AX siRNA) in response to IR (2 Gy). Quantification of the average number of DNA damage-induced BCL10 foci is shown on the right. (B) Immunodetection of BCL10 and MRE11 in ATM proficient (WT) and ATM deficient (AT) cells in response to IR (2 Gy). Quantification of the average number of DNA damage-induced BCL10 foci per cell is shown on the right.
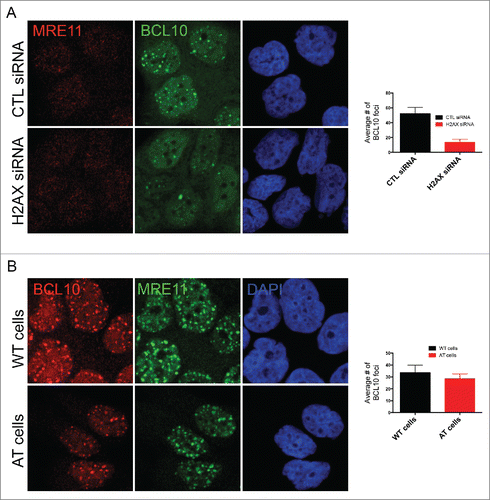
Figure 5. BCL10 regulates ubiquitylation at the sites of DNA damage. (A) Immunodetection of BCL10 and ubiquitin (FK2) in T47D cells exposed or not exposed to IR. Quantification of the average number of cryptic γ-H2AX foci per cells is shown on the right. The right panel shows Pearson's correlation coefficient values for co-localization of ubiquitin and BCL10 with and without IR. (B) Immunodetection of BCL10 and ubiquitin (FK2) in T47D cells transfected with control siRNA (CTL siRNA) or BCL10 siRNA (BCL10 siRNA) and exposed to IR (2 Gy). Arrows indicate the accumulation of ubiquitin in the cytoplasm upon BCL10 depletion. Quantification of the average number of ubiquitin (FK2) foci per cell is shown on the right. (C) Immunodetection of BCL10 and RNF8 or RNF168 in T47D cells transfected with control siRNA (CTL siRNA) or BCL10 siRNA (BCL10 siRNA). Quantification of the average number of RNF8 and RNF168 foci per cell is shown on the right. (D) Immunodetection of BCL10 foci in T47D cells transfected with control siRNA (CTL siRNA), RNF8 siRNA (RNF8 siRNA) or RNF168 siRNA (RNF168 siRNA) and exposed to IR (2 Gy). Quantification of the average number of ubiquitin BCL10 foci per cell is shown on the right.
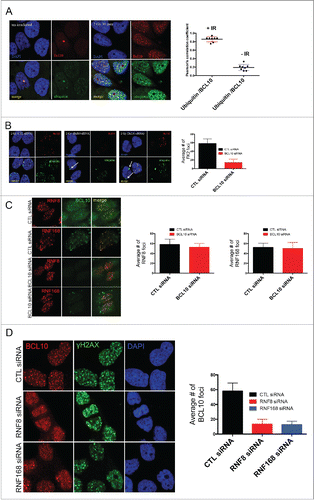
Figure 6. ATM phosphorylates BCL10 is in response to DNA damage. (A) Cells were treated with the indicated drugs and cytoplasmic and nuclear fractions were isolated. BCL10 immunoprecipitation was conducted using BCL10 antibodies. Immunoblots were performed as indicated. (B) Cells were incubated with the indicated inhibitors (20μM Wort., Wortmannin; 2μM DNA-PKi, DNA-PK inhibitor; 10 μM ATMi, ATM inhibitor) for 1 hr and then exposed to IR (10 Gy). Nuclear extracts were isolated and subjected to immunoprecipitated using BCL10 antibody. Western blot analysis was conducted with BCL10 and p-BCL10 antibodies.
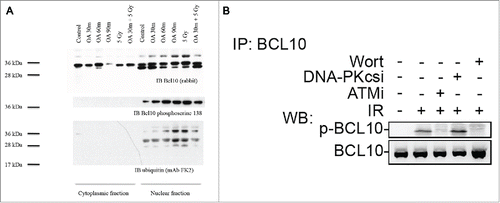
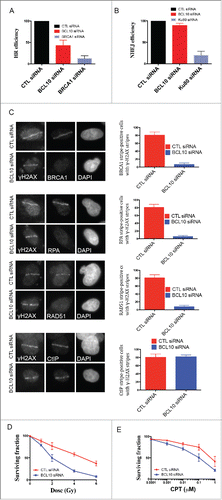
Figure 7. BCL10 promotes DSB repair and cellular sensitivity to DNA damage. Efficiency of BCL10 cellular depletion on DSB repair in cells transfected with control siRNA (CTL siRNA) or BCL10-specific siRNA (BCL10 siRNA). (A) Cells contain an integrated tandem GFP reporter of HR. The percentage of GFP positive was measured by flow cytometer. Each data point represents the mean ± the SEM of 2 separate experiments. (B) Cells contain an integrated tandem GFP reporter of NHEJ. The percentage of GFP positive was measured as in A. (C) Effect of BCL10 depletion on HR proteins. Cells were treated as in A, laser micro-irradiated and allowed to recover for 1 hr and immunostained with different antibodies as indicated. Representative pictures were shown on the right. The percentage of cells with γ-H2AX/BRCA1, γ-H2AX/RPA, γ-H2AX/RAD51 and γ-H2AX/ CtIP were shown on the right. (D and E) Sensitivity of BCL10 knock down cells to different DNA damaging agents (IR and CPT). Survival of U2OS cells upon BCL10 knockdown (BCL10 siRNA) compared with control (CTL siRNA) in response to (D) IR or (E) CPT. Error bars indicate SEM from 2 independent experiments. The results are normalized to plating efficiency.