Figures & data
Figure 1. Domain structure of (A) human LILRBs and (B) mouse orthologs. Extracellular Ig-domains are depicted as hexagons and intracellular ITIMs are depicted as boxes.
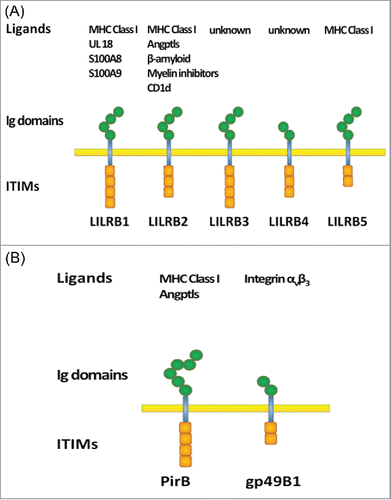
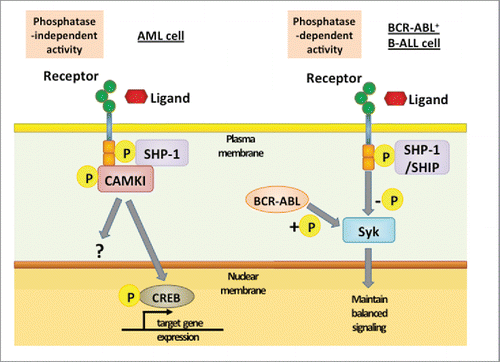
Figure 2. Downstream signaling of ITIM-containing receptor in different leukemia cells. In AML cells, binding of ligands or interaction between receptors activates LILRBs and results in tyrosine phosphorylation in ITIMs. This event is followed by recruitment of SHP-1, which acts as a phosphatase-independent scaffolding protein and forms a complex with the kinase CAMKI. CAMKI activation then induces phosphorylation and nuclear translocation of transcription factor CREB. In contrast, in BCR-ABL+ B-ALL cells, BCR-ABL-induced phosphorylation of Syk is balanced by SHP-1- and SHIP-mediated dephosphorylation. By preventing hyperphosphorylation of Syk, ITIM-containing receptors enable over-activated malignant B cells to avoid negative selection.