Figures & data
Figure 1. Mutation of E2f4 and E2f5 leads to seminiferous tubule and rete testis dilation and a failure of spermatozoa to reach the epididymis. (A) Sagittal sections of adult testis from control (Ctrl) and (B) E2f4f/f;E2f5+/−;Vil-cre littermates showing dilation of the seminiferous tubules and rete testis as well as spermatozoa within the rete testis (arrow), and inset image in the mutant. (C) Seminiferous tubules from control and (D) mutant testis showing tubule dilation. (E) Section through the rete testis of control and (F) mutant testis showing rete testis dilation. (G) The efferent ducts of control and (H) mutant testis showing spermatozoa within the efferent ducts of the mutant (arrow). (I) The epididymis of control adults contains spermatozoa in contrast with the mutants (J) where none are detected. All tissue sections were stained with hematoxylin and eosin. Scale bars in A and B, 250 μm; E and F, 200 μm; C, D, I and J, 100 μm; G and H, 20 μm.
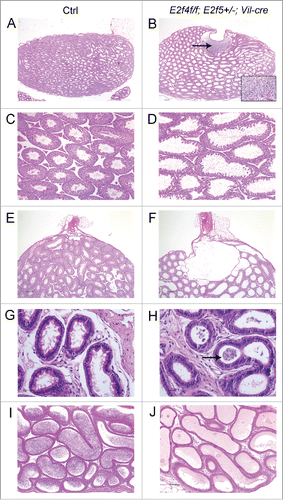
Figure 2. Efferent ducts of adult E2f4f/f;E2f5+/−;Vil-cre mutants lack multiciliated cells. (A) Cilia project into the lumen from the apical surface of multiciliaited cells in controls (Ctrl) but are absent from mutants (B), sections stained with hematoxylin and eosin. (C) Immunohistochemical staining for acetylated tubulin (brown stain) of cilia shows multiciliated cells in controls but not mutants (D). The staining within the lumen of the mutants corresponds to the flagella of spermatozoa. (E) Immunohistochemical staining for E2F4 shows nuclear staining (brown stain) in nuclei of the efferent duct epithelium in the controls but not mutants (F). (G) Immunohistochemical staining for a multiciliated cell expressed transcription factor, FOXJ1 (brown stain) shows nuclear staining in the controls and reduced expression in the mutants (H). Scale bars in C, D, G and H 20 μm; A, B, E and F 10 μm.
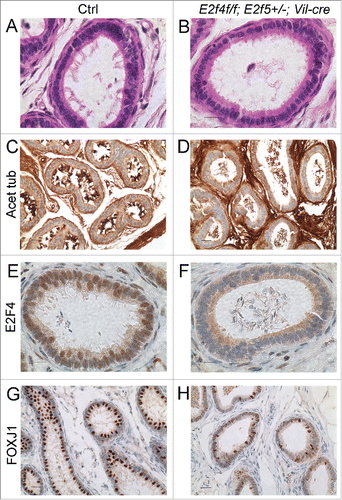
Figure 3. Expression of a multiciliated cell marker, FOXJ1 is reduced early during efferent duct development. Immunohistochemical staining for E2F4 or FOXJ1 (brown stains) in efferent ducts from one week old littermates. (A) Control efferent ducts (Ctrl) showing nuclear expression of E2F4 and (B) the loss of E2F4 from E2f4f/f;E2f5+/−;Vil-cre efferent duct epithelium at this stage. Note that mesenchymal expression of E2F4 is not lost in the mutants. (C) Robust expression of FOXJ1 in the controls in comparison with weak expression in the mutants (D). Scale bars: for each set, left hand panel 20 μm and the right hand panel 10 μm.
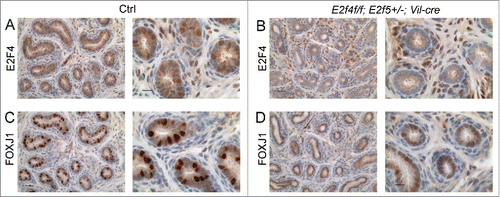
Figure 4. Efferent duct marker analyses indicates abnormal development of the adult E2f4f/f;E2f5+/−;Vil-cre efferent duct epithelium. (A) Immunohistochemical analysis of estrogen receptor 1 (ESR1) expression (brown stain) in control and (B) mutant efferent ducts showing reduced expression in the mutants. (C) Aquaproin 1 (AQP1) expression (brown stain) is predominantly at the apical surface of control efferent ducts (arrow) but poorly expressed in the mutants (D). (E) Clusterin (CLU) a marker for the endocytic apparatus is located predominantly beneath the apical surface in controls (arrow) but poorly expressed in mutant efferent ducts (F). (G) Sections submitted to the PAS reaction show apical staining in the controls but not in the mutants (H) indicating a loss of endocytic apparatus. Scale bars in all panels 10 μm.
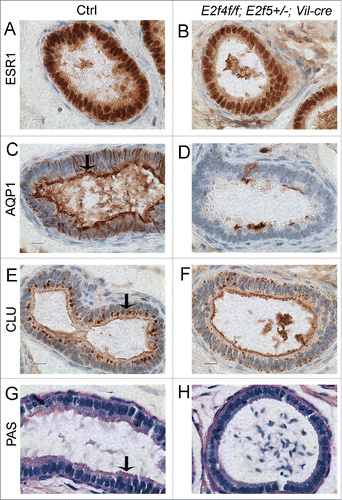
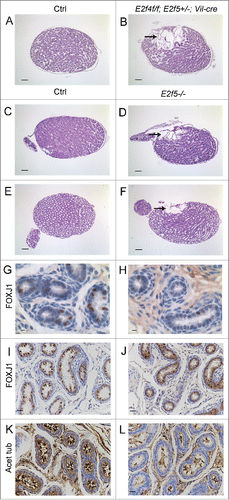
Figure 5. Dilation of the rete testis is observed at one week of age in E2f4f/f;E2f5+/−;Vil-cre and E2f5−/− testes and loss of E2F5 reduces the multiciliated cell population. (A) Sagittal sections of testis from one week old control (Ctrl) and (B) E2f4f/f;E2f5+/−;Vil-cre mutant testis showing extensive dilation of the rete testis in the mutant (arrow). (C) Sagittal sections of testis from one week old control (Ctrl) and (D) E2f5−/− mutant testis showing extensive dilation of the rete testis in the mutant (arrow). (E) Sagittal sections of testis from 3 week old control (Ctrl) and (F) E2f5−/− mutant testis showing dilation of the rete testis (arrow) and partial dilation of seminiferous tubules in the mutant. (G) Immunohistochemical staining for FOXJ1, a multiciliated cell marker in one week old control and (H) E2f5−/− mutant efferent ducts showing reduced expression in the mutants. (I) Immunohistochemical staining for FOXJ1 in 3 week old control and (J) E2f5−/− mutant efferent ducts showing reduced expression in the mutants. (K) Immunohistochemical staining for acetylated tubulin staining (brown stain) of cilia shows many multiciliated cells in efferent ducts from 3 week old controls but many fewer multiciliated cells in littermate E2f5−/− mutant efferent ducts. Panels A though F hemotoxylin and eosin stained sections. Scale bars: E and F 400 μm; A, B, C, D 200 μm; I, J, K and L 20 μm; G and H 5μm.