Figures & data
Figure 1. Isoproterenol directs the differentiation of HAP cells into cardiac muscle cells. (A) Schematic diagram of inducing conditions for cardiac muscle cell differentiation from the upper part of vibrissa hair follicles. Hair follicles were cultured for 14 days in 3 conditions in DMEM medium with 10% fetal bovine serum (FBS): unsupplemented; supplemented with isoproterenol; or supplemented with the combination of isoproterenol+activin A+BMP4+bFGF; once every four days. (B) Immunostaining of cardiac muscle cells differentiated from the upper part of vibrissa hair follicles in culture medium that was non-supplemented (left panel); isoproterenol-supplemented (middle panel); and supplemented with the combination of isoproterenol+activin A+BMP4+bFGF (right panel). Cardiac muscle cells were observed after 14 days culture. Red = cTnT, Blue = DAPI. Bars = 100 µm.
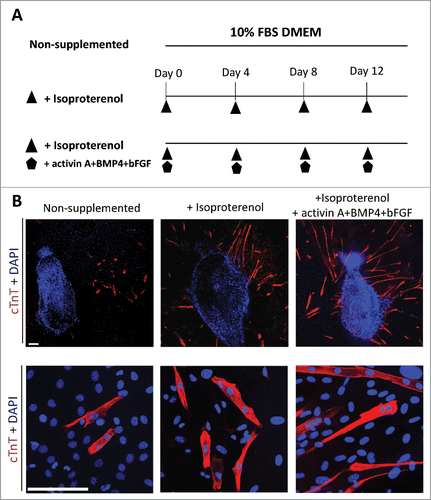
Figure 2. Flow cytometry to determine cTnT expression in HAP stem cell-derived cardiac muscle cells. (A) Flow cytometry analysis of cTnT-positive cardiac muscle cells that differentiated from the upper part of hair follicle cultured in medium that was non-supplemented at 8.78 ± 3.60% (left panel); in medium supplemented with isoproterenol alone at 19.71 ± 2.20% (middle panel); in medium supplemented with the combination isoproterenol, activin A, BMP4 and bFGF at 16.84 ± 1.67% (right panel). (B). The number of differentiated cTnT-positive cardiac muscle cells was significantly increased in the hair follicle cultured in medium supplemented with isoproterenol alone or the combination of isoproterenol, activin A, BMP4 and bFGF compared with unsupplemented medium.
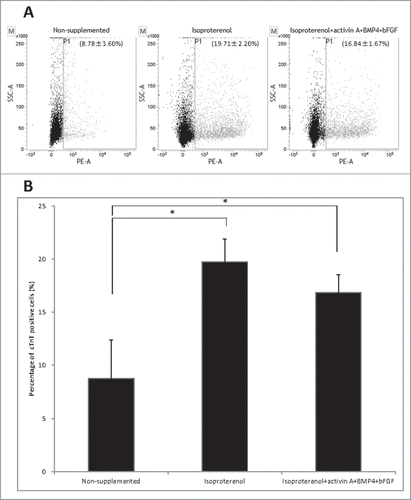
Figure 3. The combination of isoproterenol, activin A, BMP4, and bFGF induced HAP stem cells in vibrissa hair follicles to form beating cardiac-muscle tissue sheets. (A) Schematic diagram of upper parts of five hair follicles forming cardiac-muscle tissue sheets (upper panel). Bright-field image of upper part of hair follicle differentiatimg to cardiac muscle cells and immunostaining (middle panel). Red = cTnT, Blue = DAPI, Bars = 100 µm. Cardiac-muscle tissue sheet formation process (lower panel). Bar = 500 µm. (B) Immunostaining of beating cardiac-muscle tissue sheets. cTnT-positive cardiac muscle cells were extensively distributed within the sheets. Red = cTnT, Blue = DAPI. Bars = 100 µm.
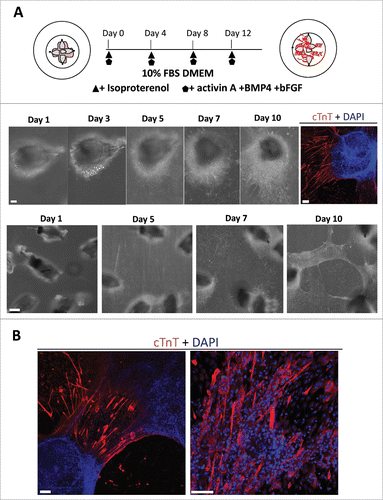
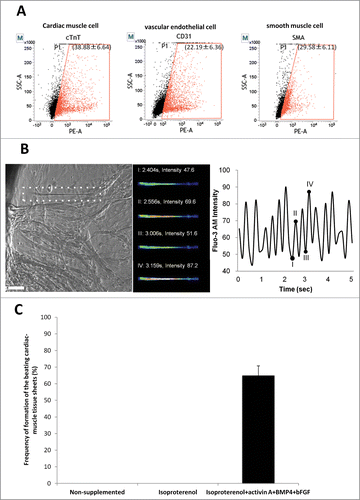
Figure 4. Flow cytometry analysis of beating cardiac-muscle tissue sheets. (A) Flow cytometry analysis of harvested cardiac-muscle tissue sheets showed that the sheets contain 38.88 ± 6.64% cTnT-positive cells (left panel); 22.19 ± 6.36% CD31-positive cells (middle panel); and 29.58 ± 6.11% SMA-positive cells (right panel). (B) Intercellular Ca2+ imaging of cardiac-muscle tissue sheets. Phase contrast image of cardiac muscle cells loaded with Flou-3 (white dashed area). Images were obtained every 150 msec (left panel). Bar =100 µm. Fluo-3 image at 4 points (I, II, III, IV) showing time course of Fluo-3 intensity change (middle and left panel). (C) High rate of formation of the beating cardiac-muscle tissue sheets from HAP stem cells. Hair follicles were cultured in medium supplemented with isoproterenol, activin A, BMP4 and bFGF, where cardiac-muscle tissue sheets formed at 65.0 ± 5.77%. No cardiac tissue sheets formed in non-supplemented medium or medium supplemented with isoproterenol alone.