Figures & data
Figure 1. CD4cre:PP4f/f mice exhibit defective T-dependent immune responses in vivo. (A) Mice at 6–8 wk age were immunized with OVA/CFA emulsion in the foot pad; one wk later lymphocytes from the draining LN were assessed in vitro by CFSE dye dilution for OVA-induced T cell proliferation (n = 8). (B) Day 3 culture supernatants from cells re-stimulated with 3 μg/ml OVA in (A) were subjected to multiplex assay to measure Th1/Th2 cytokines secretion (n = 6). (C) Mice at 6–8 wk age were immunized i.p. with the indicated epitope/antigen/adjuvant, and their sera were collected on d 21 for primary Ig responses (top panels). Mice were immediately boosted by immunization and their sera collected again on d 35 for memory Ig response (bottom panels). (n = 3–4). AU, arbitrary unit. *, p < 0.05; **, p < 0.01; ***, p < 0.005. See Supplemental Figure S1A for flow cytometry gating strategies.
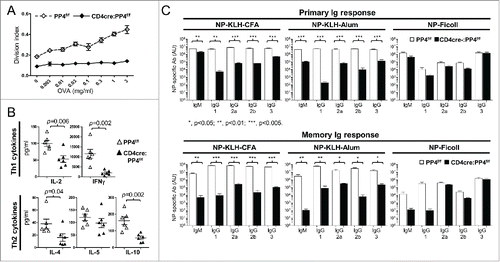
Figure 2. The ablation of PP4 impairs T cell expansion. (A) Schematics of the genomic qPCR assay for quantifying ppp4c deletion efficiency. a-d, PCR primers. Triangle, lox-P sites. (B) CD4, CD8 or control CD4−CD8− splenocytes or MLN cells were sorted by flow cytometry from mice at different ages; DNA extracted from these cells was analyzed by qPCR as in (A) for assessing the deletion efficiency of ppp4c (n = 2–3). (C) Schematics of the homeostatic expansion assay (left panel) and the representative CD90.2 histogram of CD4 splenocytes from the recipient (right panel) are shown. (D-E) WT (CD90.2−) or CD4cre:PP4f/f (CD90.2+) total T cells were purified from pooled spleen and LN by MACS, mixed at a 1:1 ratio (day 0), labeled with CFSE, and transferred into RAG1−/− mice. At day 1 or day 8 post-transfer, spleen, ALN or MLN cells from the recipients were analyzed by flow cytometry for the percentages of WT or CD4cre:PP4f/f donor cells and their respective CFSE dye-dilution patterns. The relative percentages of WT and CD4cre:PP4f/f donor cells (left panels) in gated CD4+ (D) or CD8+ (E) populations are shown. Representative CFSE dye dilution profiles of WT and CD4cre:PP4f/f donor cells are also shown (right panels) (n = 3). See Supplemental Figure S1A–B for flow cytometry gating and sorting strategies.
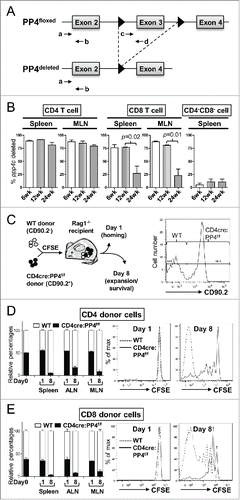
Figure 3. The hypo-proliferation of PP4-deficient CD4 T cells coincides with a partial G1-S block, and is mediated primarily via cell-intrinsic mechanisms. (A-B) Total T cells were purified by MACS, labeled with CFSE, stimulated with a non-saturating dose of 1.6 μg/ml anti-CD3ε and anti-CD28, and analyzed daily by flow cytometry. Representative CFSE profiles (panel A) and division indexes calculated by the FlowJo software (panel B) of gated CD4 cells are shown (n = 6–7). (C-D) MACS-purified T cells were activated as in panel A in the presence of 10 μM BrdU; at the specified time, cells were fixed and analyzed for cell cycle status by intracellular staining of BrdU and 7AAD. Representative FACS plots and gate nomenclatures (panel C), as well as statistical analyses of cell cycle status (panel D) from CD4 T cells are shown (n = 4–5). (E-F) Schematics and results of the competitive proliferation assay. MACS-purified WT and CD4cre:PP4f/f T cells were mixed at 1:1 ratio, labeled with CFSE, and activated and analyzed as in panel A (panel E). The percentages of WT or CD4cre:PP4f/f T cells in gated CD4 populations are shown (n = 6–7) (panel F). See Supplemental Figure S1A, -C and-D for flow cytometry gating strategies.
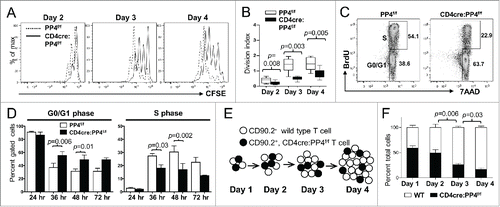
Figure 4. Reduced IL-2 production or signalings is not the primary cause for the hypo-proliferation of PP4-deficient T cells. (A) Day 3 culture supernatants from were analyzed by multiplex assays for the secretion of various Th1/Th2 cytokines (n = 5–6). (B) MACS-purified, CFSE-loaded T cells were activated by sub-optimal 0.5 μg/ml plate-bound anti-CD3ε and anti-CD28 in the presence or absence of exogenous IL-2; this was then followed by the analyses of CD25 induction (left panel) or proliferation (right panel) on gated CD4 populations (n = 2–3). (C) MACS-purified T cells were activated by 1.6 μg/ml anti-CD3ε and anti-CD28 for 2 d, washed, and rested for 4 hr. Rested WT and CD4cre:PP4f/f cells were then treated with titrating doses of IL-2 and stained intracellularly for phospho-STAT5-Y694. Representative flow cytometry plots (left panels) and statistical analyses of the mean fluorescence levels (right panel) are shown (n = 2–4). (D) JTAg cells were transfected with the respective luciferase reporter plasmids and control tdTomato plasmid by electroporation; cells were stimulated 24 hr later with PMA/ionomycin and various doses of OA for 6 hr prior to luciferase activity measurement, as well as FACS analyses for assessing the transfection efficiency by tdTomato fluorescence. Luciferase activity after compensating for transfection efficiency and normalized to the no-OA samples (Calibrated light unit) are shown (n = 3). See Supplemental Figure S1A and -E for flow cytometry gating strategies.
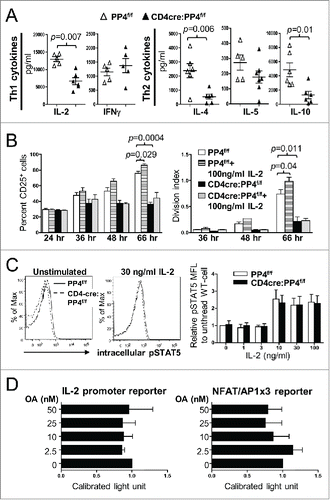
Figure 5. The partial G1-S block induced by PP4 deficiency is associated with enhanced expression of CDK inhibitors p16/Ink4a and p15/Ink4b. (A, D-F) RNA was collected from T cells activated as in at the specified time for qPCR analyses. Fold inductions relative to unstimulated WT T cells, after compensating for signals from β–actin, are shown for the respective genes (n = 3–7). (B-C) MACS-purified T cells were activated as above with their lysates collected at the indicated time for western blot analyses. Representative protein gel blotting results are shown (n = 3–5). 3 + 28, anti-CD3ε+anti-CD28 stimulation.
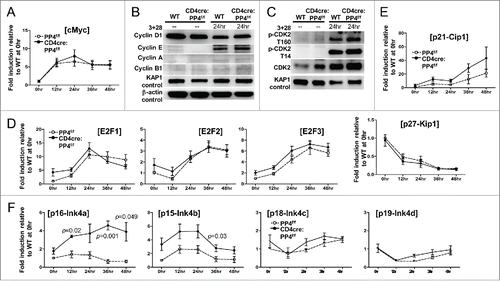
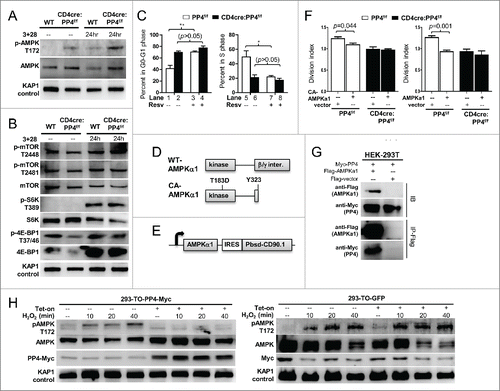
Figure 6. AMPK hyper-activation is induced by PP4 ablation and correlates with retarded T cell proliferation. (A-B) MACS-purified T cells were activated and analyzed by western blot analyses as in for AMPK activation (panel A), or mTOR activation and mTOR substrate phosphorylation (panel B). Representative protein gel blotting results are shown (n = 3). 3+28, anti-CD3ε+anti-CD28 stimulation. (C) MACS-purified T cells were activated as in in the presence or absence of 20 μM Resv and 10 μM BrdU. After 48 hr, cells were fixed and analyzed for cell cycle status by intracellular staining of BrdU and 7AAD. Statistical analyses results of gated CD4 T cells are shown (n = 4–5). (D) Schematics of WT- and CA-AMPKα1. (E) Schematics of the bicistronic lentiviral expression vector. IRES, internal ribosome entry site. Pbsd-CD90.1, blasticidin-resistant and CD90.1 fusion gene. (F) MACS-purified T cells were CFSE-loaded and activated as in in the presence of control lentivirus (vector, with CD90.1 infection marker only) or lentivirus with CA- (left panel) or WT-AMPKα1 (right panel) plus CD90.1 marker for 48 hr. Division indexes of the infected, CD90.1+ CD4 T cells are shown (n = 3–5). (G) Myc-tagged PP4 and flag-tagged AMPKα1 or control flag vector were transiently co-expressed in HEK-293T cells for 24 hr; this was then followed by lysate collection and anti-flag immunoprecipitation. Representative western blotting results from pre-immunoprecipitated lysates (IB, top half) and anti-flag-precipitated fractions (IP-Flag, bottom half) are shown (n = 3). (H) HEK-293 clones with tetracycline-inducible expression of myc-tagged PP4 (293-TO-PP4-Myc, left panel) or GFP (293-TO-GFP, right panel) were treated with 2 μg/ml tetracycline (Tet-on) for 24 hr; this was then followed by the addition of 1 mM H2O2 for the indicated time. Representative protein gel blot results from 3 independent clones are shown (n = 3). See Supplemental Figure S1F for flow cytometry gating strategies.