Figures & data
Figure 1. ARTD1 knockdown leads to cell cycle delay in the T24 hyperphosphorylated cell line. A) Western blot analysis with extracts of confluent T24 and U2OS cells to assess the levels of Rb phosphorylation. B) Western blot confirmation of ARTD1 depletion upon siRNA treatment of T24 cells. C) Flow cytometry analysis of siMock and siARTD1-treated, synchronized T24 cells at different time points after release from G0 phase and asynchronous T24 cells. D) Quantification of cell cycle analysis after knockdown of ARTD1 (A1) shown in C. Data represent mean ± SD of n = 4 independent experiments and was analyzed by 2-way-ANOVA followed by a Bonferroni post-test for the G1 and S phase. *P < 0.05; ***P < 0.001: A1 statistically different to corresponding siMock (M)-treated cells at the same time point. E) Flow cytometry analysis for shMock and shARTD1 transduced T24 cells shortly after viral transduction. F) Immunofluorescence microscopy of PAR formation of untreated or H2O2-treated (10 min, 1 mM) samples, pre-treated with olaparib (1 µM) or veliparib (1 µM). G) Inhibition of the enzymatic activity leads to an S phase arrest. Overlay of flow cytometry samples of T24 cells: untreated (non-transduced), olaparib (24 h, 1 µM) and veliparib (24 h, 1 µM) treated samples (24 h time point).
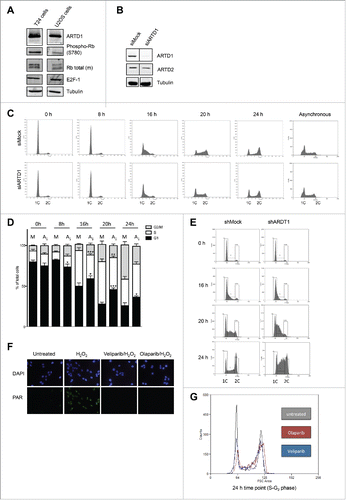
Figure 2. Cyclin E protein levels are reduced upon ARTD1 depletion. A-B) Western blot analysis of p53 (A) and γH2AX (B) after cell cycle re-initiation of synchronized siMock (M) or siARTD1 (A1) treated T24 cells at different time points. As positive control (T24*/U2OS), cells treated with etoposide (10 µM, 16 h) were used. C) qPCR analysis of cyclin A, B and D1 in siMock and siARTD1-treated cells. (n = 2) D) qPCR analysis of cyclin E in siMock and siARTD-treated T24 cells (n = 4, t-test). E) Western blot analysis of cyclin E levels in siMock and siARTD1 treated cells. F) qPCR analysis of cyclin E expression in cells treated with the PARP inhibitor olaparib 1 μM (n = 2). (G) qPCR analysis of asynchronous siMock- and siARTD1-treated T24 cells.
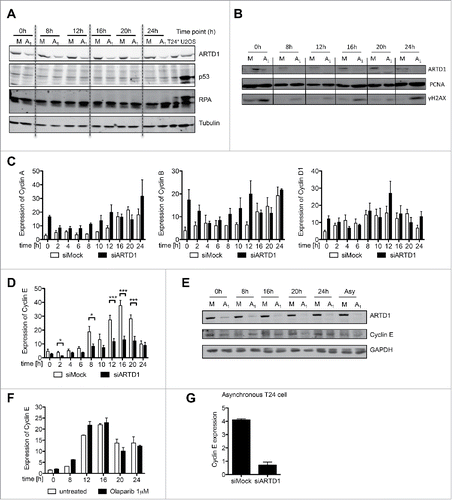
Figure 3. ARTD1 is recruited to the cyclin E promoter and keeps the chromatin in an open conformation. A-B) qPCR analysis (n = 4, t-test) (A) and Western blot analysis (B) of E2F-1 in synchronized siMock and siARTD1-treated T24 cells. C) ChIP analysis of E2F-1 antibody binding to the cyclin E promoter in T24 cells during cell cycle progression. D-I) ChIP analysis of the cyclin E promoter in siMock and siARDT1-treated T24 cells. ARTD1 binding (D), and H3 occupancy (E), H3K4me3 (F), H4 acetylation (G), H2AZ occupancy (I), and H1.2 occupancy (J) during the G1 phase (10 h time point) were analyzed.
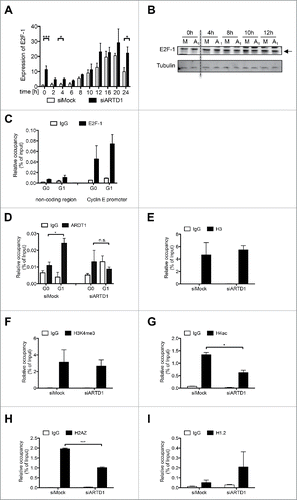
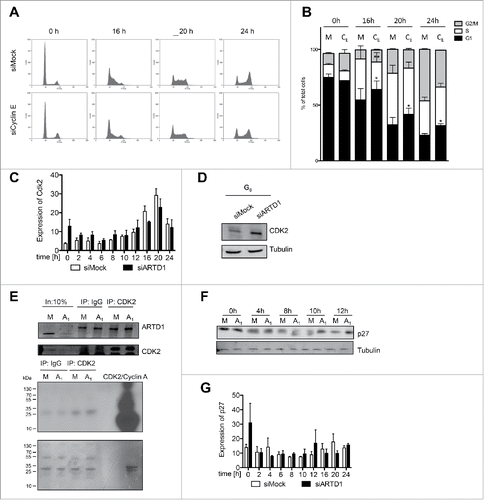
Figure 4. Reduced functional activity of the cyclin E/Cdk2 complex and the consequent increase in p27 levels account for the reduced cell cycle entry in ARTD1-depleted T24 cells. A) FACS analysis of synchronized siMock, siARDT1 and siCyclin E-treated cells during cell cycle progression. B) Quantification of cell cycle analysis after knockdown of cyclin E (CE) shown in C. Data represent mean ± SD of n = 3 independent experiments and was analyzed by 2-way-ANOVA followed by a Bonferroni post-test for the G1 and S phase. *P< 0.05; **P<0.01 CE statistically different to corresponding siMock (M)-treated cells at the same time point. C) qPCR analyses of Cdk2 in siMock and siARTD1-treated samples (n = 4). D) Western blot analysis of Cdk2 in synchronized siMock (M) and siARTD1 (A1)-treated cells. E) Activity assay of Cdk2-cyclin E, immunoprecipitation of Cdk2 at 12 h after re-entry into the cell cycle (upper panel) and radioactive assay with immunoprecipitated Cdk2 or IgG control. Radioactive ATP was used for the assay (lower panel). F) Western blot analysis of p27 protein levels in control and siARTD1 samples (n = 2). G) qPCR analysis of p27KIPin siMock and siARTD1-treated samples.