Figures & data
Figure 1. Aneuploidy is increased and has no effect on GVBD and PBE in aged oocytes. (A) Numbers analyzed are indicated above the bars. The data were analyzed using a chi-square test. The experiments were repeated more than 3 times. ** P < 0.01. (B) Kinetics of germinal vesicle breakdown (GVBD). GV-stage oocytes were isolated in M2 medium containing dbcAMP, which inhibits GVBD, and were released into inhibitor-free medium (at time = 0). The number of oocytes examined is indicated (n). (C) Kinetics of polar body extrusion (PBE). Oocytes that had undergone GVBD within 1.5 h after release into dbcAMP-free M16 medium were selected (at time = 0). The number of oocytes examined is indicated (n).
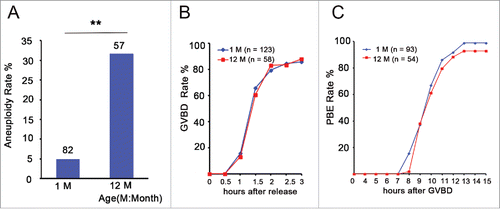
Figure 2. Aging leads to an increase in oocyte pHi. (A) Full-grown and denuded GV oocytes were incubated with BCECF-AM to determine the pHi. Scale bar, 30 μm. (B) Full-grown GV oocytes were released from the ovaries of mice of different ages and were then removed to low-lactate KSOM to stabilize for 15 min before their pHi values were measured. (C) Oocytes from 1- and 12-month-old mice represent the young and aged groups, respectively. Full-grown GV oocytes were cultured in M16 medium for 2-2.5, 7.5-8 and 16-17 h to reach GVBD, MI and MII stages, respectively. In B and C, each point represents between 47 and 120 oocytes from 4 to 8 replicates. Data are presented as the means ± SEM.
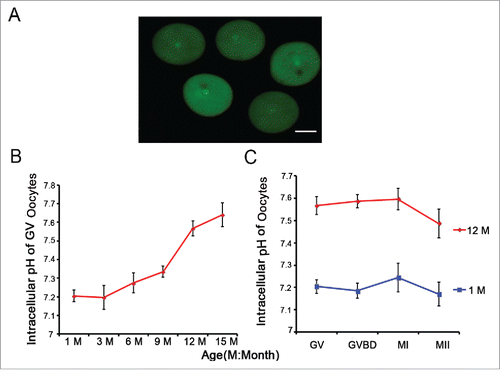
Figure 3. HCO3−/Cl− exchanger activity is attenuated with advancing age in full-grown GV oocytes. (A) Model for the increase in oocyte pHi using a Cl− removal medium. (B) pHi was monitored in denuded and full-grown GV oocytes from 1-, 3-, 6-, 9-, 12-, and 15-month-old mice; simultaneously, Cl− was removed from the bathing medium at 10 mins, indicated by the red arrow; Mins: minutes, M: month. Traces shown are the means of all experiments performed. (C) The rate of pHi increase upon C− removal in GV oocytes was quantified, providing an indication of HCO3−/Cl− exchanger activity. Each point represents between 47 and 120 oocytes from 4 to 8 replicates. (D) The relative AE2 mRNA expression levels in GV oocytes from 1- and 12-month-old mice. Gapdh served as the internal control gene. The data are expressed as the means ± SEM. ** P < 0.01. Each experiment employed 80 oocytes and was repeated 4 times.
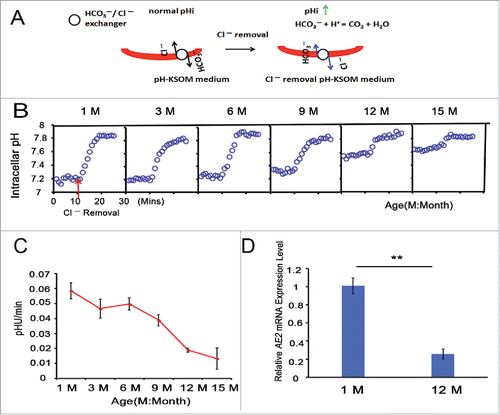
Figure 4. Alkaline treatment of young oocytes causes increases in chromosome aneuploidy and sister iKT distance. (A) Model for the process of alkaline treatment of young oocytes. 1) The pHi of GV oocytes was increased. 2) The increased pHi was maintained in different oocyte phases. (B) MII oocytes were spread in situ. A flattened z-stack series of images through a monastrol-treated egg (middle). Chromosomes were counted using a flattened z-stack series of images; the white circle represents a sister chromatid pair (right). Representative sister chromatid pairs from the normal control, DIDS control and alkaline treatment groups illustrating the increase in iKT distances; the white line shows the inner distance of the sister chromatid pair (left). Red, centromere (CREST); blue, chromatin (4,6-diamino-2-phenyl indole, DAPI). Bar = 5 μm. Alkalinization: oocytes were matured in a medium supplemented with DIDS after being removed to Cl−-free media for a 10-min treatment. Normal control: oocytes were matured in media without any addition and treatment. DIDS control: oocytes were matured in media supplemented with DIDS but without Cl−-free media treatment. In C and D, the numbers analyzed are indicated above the bars; ** P < 0.01; * P < 0.05. In each experiment, the data shown represent more than 3 replications.
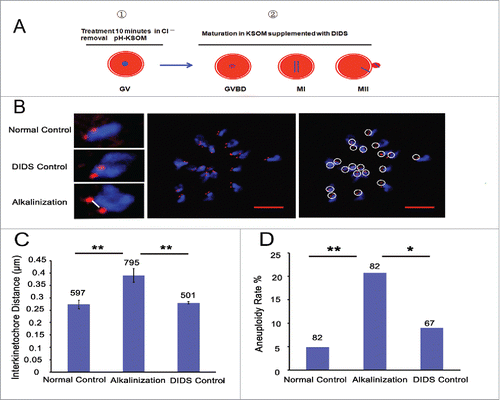
Figure 5. The expression level of SMC3 protein on chromosomes was obviously decreased in alkaline young oocytes. (A) The levels of chromosome-associated SMC3 protein were detected by immunofluorescence in normal, DIDS control and alkaline-treated oocytes at MI. Representative images show DNA (blue), CREST (red) and SMC3 (green). Scale bar, 5 μm. (B) SMC3 fluorescence intensity was quantified. Data are the means ± SEM, ** P < 0.01; ≥150 bivalents of each group were analyzed.
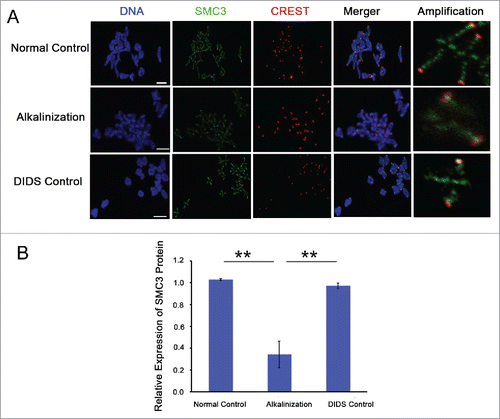
Figure 6. Chromosome misarrangement and normal KT-MT attachment in alkaline oocytes. (A) Chromosomes and spindles were labeled with H2B-red and Map7-green, respectively. The images of chromosomes and spindles were captured at 30-min intervals over 16-17 h. The time of anaphase I onset was marked with a red square. Chromosom misarrangement was marked with a red arrow. Movies for the normal control and the alkalinization-treated groups are available (Supplemental Videos S1, S2). Scale bar, 10 µm. (B) Chromosome misalignment is shown in a model and marked with a black triangle. Oocytes with misaligned chromosomes were confirmed at the onset of anaphase I. The data were analyzed using a chi-square test. (C) Representative images of the 3 groups are shown. The iKT distance of misaligned sister pairs was more than 10 μm, and its one kinetochore had an amphitelic attachment (i). Misaligned sister pairs had a single polar attachment, marked with a white arrow (ii). DNA: blue, kinetochore: red, α-tubulin: green. Scale bar, 10 µm. (D) Analysis of attachment types in oocytes from the normal control, DIDS control and alkalinization-treated groups. A total of 800 univalent attachments from 20 normal-control oocytes, 840 univalent attachments from 21 DIDS-control oocytes, and 1000 univalent attachments from 25 alkaline-treated oocytes were classified from 3 experimental replicates. Each attachment type was shown using white arrow.
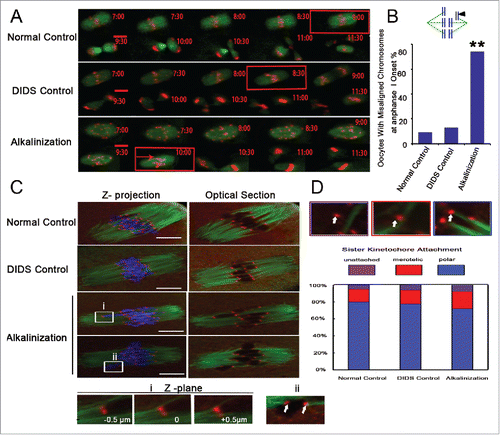
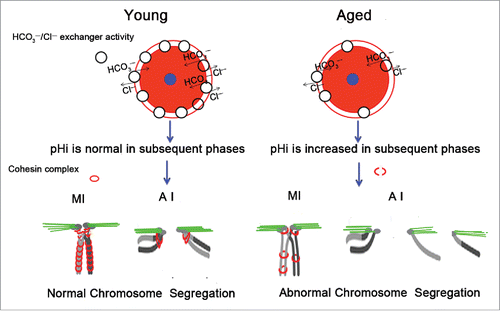
Figure 7. Schematic representation of the involvement of increased pHi in aneuploidy in old oocytes. Aging causes decreased HCO3−/Cl− exchanger activity in full-grown GV oocytes, which have the strongest HCO3−/Cl− exchanger activity of the oocyte phases.Citation26,27 Therefore, in subsequent phases of aged oocytes, anions cannot be excluded in a timely manner to the extracellular compartment due to reduced activity of the HCO3−/Cl− exchanger, leading to an increase in the oocyte pHi. The increased pHi of aged oocytes might affect the cohesin subunit-subunit binding affinity, change its stability, modify its function, and alter its subcellular localization, among other effects. Finally, the increased pHi of aged oocytes gives rise to the deterioration of the cohesin wrapped around the chromosome, leading to increased aneuploidy. The small blue circle represents the nucleus, and the pink circle represents the cytoplasm.