Figures & data
Figure 1. Pten disruption results in polyploidy and nocodazole resistance. (A and B) Flow cytometry analysis of ploidy distribution and DNA content in Pten+/+ and Pten−/− MEFs. Data are presented as mean ± SEM of 3 independent experiments (*, p < 0.05; as compared with Pten+/+ cells with the same ploidy population). (C) Karyotypic profiles of Pten+/+ and Pten−/− MEFs with or without spindle disruption by nocodazole (1.5 μM). Data are summarized from 3 independent experiments and analyzed with the paired t-test. *, p < 0.05; **, p < 0.01; as compared with corresponding untreated or nocodazole treated Pten+/+ cells. (D) Growth curves for Pten+/+ and Pten−/− MEFs in response to nocodazole (1.5 μM). (E) HeLa cells were transfected with pSuper/sh PTEN and PTEN expression was evaluated by Western blotting and compared with cells containing a control pSuper/sh control vector. (F) Immunofluorescent staining of microtubules using an anti-α-tubulin antibody and visualized with an FITC-conjugated species-matched secondary antibody. White arrowheads and red arrows indicate giant cells containing a single enlarged nucleus and multiple nuclei respectively. (G) A representative comparison of cell cycle and ploidy profiles in thymidine synchronized HeLa cells containing PTEN shRNA or scrambled shRNA, at a time point 12 hours after release from double-thymidine block (DTB). (H) Survival curves for PTEN-knockdown and control HeLa cells treated with 1.5 μM nocodazole for 24 h and 48 h.
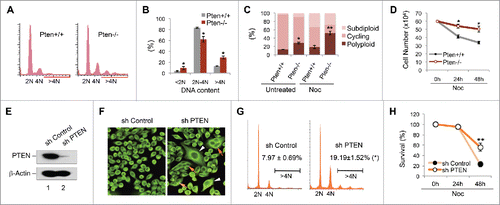
Figure 2. PLK1 is a PTEN-associated mitotic protein. (A) Expression of FH-PTEN and endogenous PTEN in FH-PTEN-expressing HeLa cells. Western blot analysis of HeLa cells containing FH-PTEN or a control vector with an anti-PTEN (upper panel) or anti-FLAG (lower panel) antibody. (B) Identification of Plk1 as a component of PTEN-associated protein complexes by pull-down assay. PTEN-complexes from HeLa/FLAG-HA-PTEN cells and a control elution from HeLa cells containing an empty vector were separated on SDS-PAGE following tandem affinity purification. A ∼70-kDa band was analyzed by mass-spectrometry and revealed PLK1 as a potential PTEN-associated protein. (C) Direct interaction of PTEN with PLK1 in vitro. Sf9 cells were infected with indicated recombinant baculovirus for 72 hours and subsequently harvested for protein purification using either an Ni-NTA agarose column or an anti-FLAG M2 affinity gel. Purified His-tagged proteins were incubated with purified FLAG-PLK1 protein followed by precipitation with Ni-NTA beads. Samples were then subjected to analysis of FLAG-PLK1 expression (upper panel). His-tagged PTEN and control protein in these reactions were shown in the lower panel. (D) Physical association of endogenous PTEN and PLK1. HeLa cells as well as MEFs with or without Pten deletion were subjected to nocodazole treatment (50 nM, 8 h) followed by immunoprecipitation with a rabbit anti-PTEN antibody and subsequent evaluation for PLK1 and PTEN by Western blotting. An isotype-matched mouse IgG was used as a control for immunoprecipitation and ExactaCruz reagents were used to reduce the interference of IgG bands. (E) Cell cycle-dependent PTEN-PLK1 association during cell division. HeLa cells were synchronized by DTB and released after different times as indicated. The interaction between PTEN and PLK1 was examined by PTEN immunoprecipitation for detection of PTEN-associated PLK1 by Western analysis. Cyclin B expression in synchronized pre-IP samples is shown to indicate mitotic entry and exit.
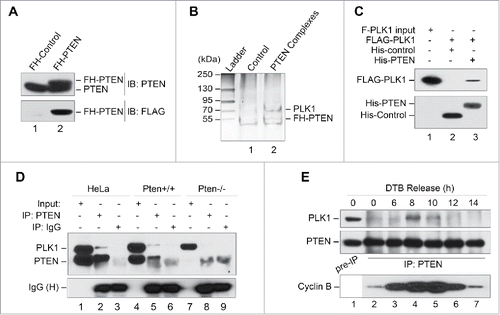
Figure 3. PLK1 is a protein substrate of PTEN phosphatase. (A and B) Pten depletion induces Plk1 phosphorylation. Pten+/+ and Pten−/− MEFs cultured with or without nocodazole (50 nM, 8 h, A), as well as various tissues from mice with wild-type Pten or heterozygous Pten deletion (B), were analyzed for Plk1 phosphorylation and abundance by immunoblotting with site-specific phospho-Plk1 (Thr210) and total Plk1 antibodies. β-Actin was used as a loading control. (C) PTEN directly and dose-dependently dephosphorylates PLK1. Sf9-expressed His-PTEN protein was purified using a Ni-NTA agarose column. FLAG-PLK1 expressed in Sf9 cells was immunoprecipitated with anti-FLAG M2 beads and incubated with increasing amounts of His-PTEN proteins, followed by Western blot analysis of PLK1 phosphorylation. The same blot was probed with PLK1 antibody to show PLK1 levels loaded in each lane. (D) Ectopic PTEN suppresses PLK1 kinase activity. Phosphorylation of NudC, a substrate of PLK1 kinase, was analyzed by immunoblotting in Pten−/− MEFs containing ectopic PTEN in the presence and absence of PLK1T210D, an enzymatically active form of PLK1.
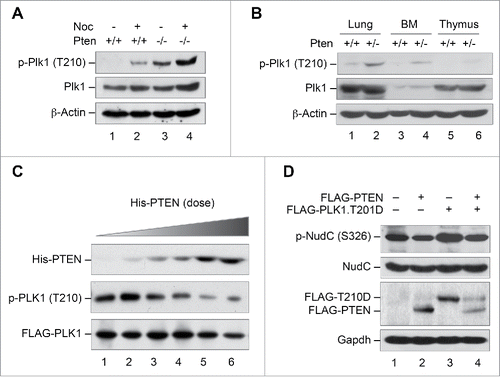
Figure 4. PTEN reduces PLK1 phosphorylation, PLK1 stability and polyploidy in a phosphatase-dependent manner. (A) Pten−/− MEFs were transfected with vectors encoding FLAG-tagged wild-type PTEN or the phosphatase-deficient PTEN mutants PTENC124S and PTENG129E, and subjected to immunoprecipitation with anti-Plk1 antibody followed by evaluation for phospho-PLK1 (Thr210). The expression of PLK1 and β-actin was then evaluated by re-blotting with corresponding antibodies. (B) Cell lysates identical to those in (A) prior to immunoprecipitation were processed for immunoblot analysis of phospho-Akt (Ser473). Endogenous and ectopic FLAG-PTEN expression was evaluated using anti-PTEN and anti-FLAG monoclonal antibodies respectively. Equal protein loading was demonstrated by re-probing the same blot with anti-β-actin antibody. (C) Pten null MEFs were co-transfected with FLAG-tagged PLK1 and different forms of PTEN (wild-type or phosphatase-deficient mutants) prior to analysis of FLAG-PLK1 expression. (D) Pten+/+ MEFs as well as Pten−/− MEFs containing wild-type PTEN, or one of the 2 phosphatase-deficient mutants (PTENC124S and PTENG129E) were cultured for 48 h followed by flow cytometric analyis of ploidy status and analyzed with the paired t-test for statistical significance. *, p < 0.05; **, p < 0.01. (E) Pten−/− MEFs containing wild-type PTEN, PTENC124S or PTENG129E were treated with 1.5 μM nocodazole for 24 h and subjected to survival analysis by trypan blue exclusion. *, p < 0.05.
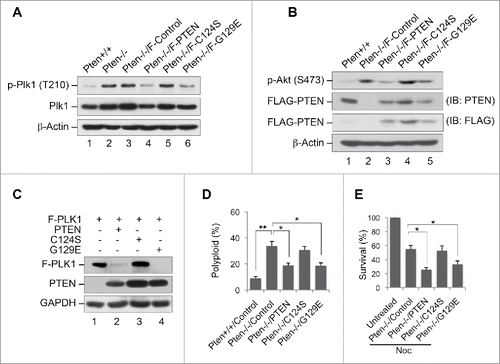
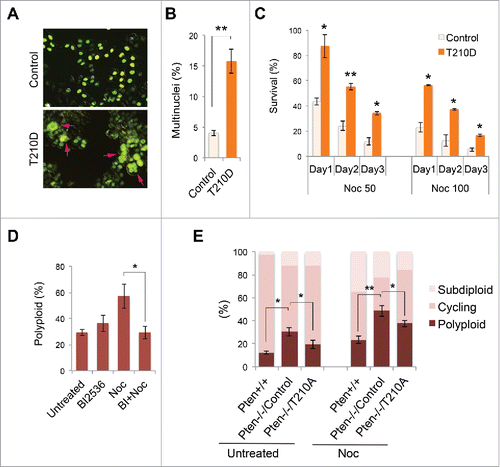
Figure 5. Constitutively active PLK1 induces multinucleation whereas inhibition of PLK1 mitigates polyploidy. (A) HeLa cells were co-transfected with GFP-labeled histone H2B as well as PLK1T210D or a control expression vector, followed by immunofluorescent microscopic analysis of cell morphology. Arrows indicate multinucleated cells. (B) HeLa cells with or without PLK1T210D (n ≥ 400) were analyzed in randomly selected regions and the percentage of multinucleated cells was plotted on the histogram. **, p < 0.01. (C) Cells with or without PLK1T210D were treated with different doses of nocodazole and cell survival was examined by trypan blue exclusion at various times during a 3-day course. *, p < 0.05; **, p < 0.01; as compared with control cells analyzed on the same day. (D) Pten−/− MEFs were pre-treated with BI 2536 (100 nM, 30 min) prior to nocodazole treatment (1.5 μM) for 48 h. Cells were evaluated for polyploidization by flow cytometry. *, p < 0.05. (E) Pten−/− MEFs transiently transfected with PLK1T210A or an empty plasmid were used as controls and were treated with nocodazole (1.5 μM) for 48 h followed by ploidy analysis. *, p < 0.05; **, p < 0.01.