Figures & data
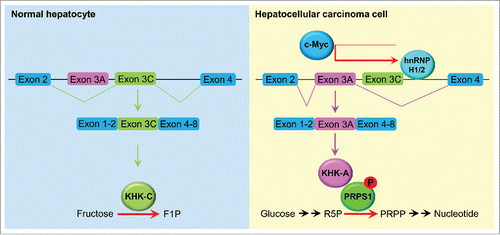
Figure 1. A splicing switch from KHK-C to KHK-A promotes HCC formation. KHK-C, the isoform of KHK with high activity toward fructose phosphorylation, was predominantly expressed in normal hepatocytes. In HCC cells, high expression of c-Myc enhanced the expression of hnRNPH1 and hnRNPH2, which bound to the exon 3C-3'/intron region of KHK, leading to a splicing switch from KHK-C to KHK-A and reduced fructose metabolism. KHK-A phosphorylated and activated PRPS1, resulting in increased de novo nucleic acid synthesis for hepatocellular tumorigenesis. F1P, fructose 1-phosphate; R5P, ribose-5-phosphate; PRPP, phosphoribosyl pyrophosphate.