Figures & data
Figure 1. Identification of post-translational modification sites of YAP1-2 gamma. Mass spectrometry analysis identified the potential YAP1 phosphorylation sites. The peptide sequences of YAP1 recovered by mass spectrometry are labeled in yellow; the serine and threonine phosphorylation sites are labeled in green; the tyrosine phosphorylation site was labeled by red.

Figure 2. Examination of endogenous YAP1 tyrosine phosphorylation in breast cancer cells. (A) Expression of tyrosine-phosphorylated YAP1 and total YAP1 in a panel of breast cancer cells as examined by immunoblot. β-actin was used as a loading control. (B) Tyrosine phosphorylation of- YAP1 induced by EGF treatment in MCF10A cells.
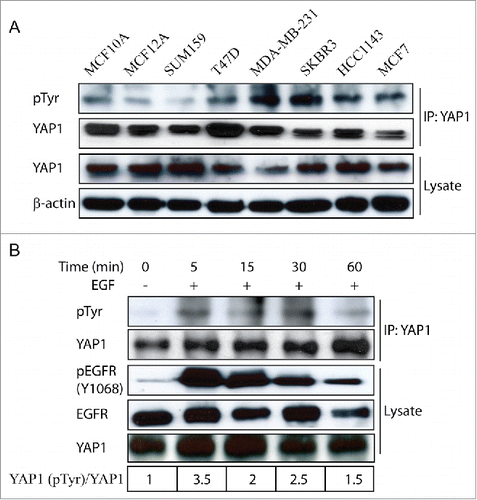
Figure 3. YAP1-Y188E enhanced the YAP1 oncogenic functions. (A) Schematic representation of YAP1. The 6 tyrosine sites of YAP1 are indicated. (B) The Y-F mutations have no effect on the protein expression of YAP1, as shown by immunoblot in transduced MCF10A cells. β-Actin was used as a loading control. (C) Overexpression of YAP1-Y188F obliterates the aberrant acinar formation induced by YAP1 in 3D culture. Representative phase contrast images from 3 independent experiments are shown. (D) Expression of YAP1-WT, YAP1-Y188F and YAP1-Y188E at comparable levels in transduced MCF10A cells as revealed by immunoblot. β-Actin used as a loading control. (E) YAP1-Y188E overexpression increased cell migration. MCF 10A cells transduced with vector control or YAP1 variants were plated onto 8-µm transwell filters and allowed to migrate for 24 hrs. (***, p < 0.001) (F) YAP1-Y188E overexpression enhanced the colony formation in soft agar.
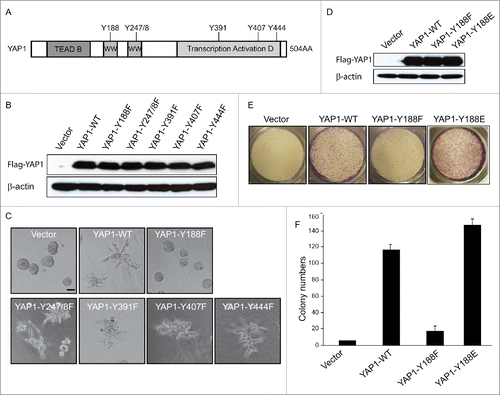
Figure 4. YAP1-Y188F fails to rescue the loss-of-function of YAP1 in MDA-MB-231 breast cancer cells. (A) Immunoblot demonstrates effective knockdown of YAP1 with the lentiviral shYAP1 construct in MDA-MB-231 cells. β-Actin was used as a loading control. (B) Expression of shYAP1-resistant WT-, Y188F- and Y188E-YAP1 variants in the MDA-MB-231 cells with stable knockdown of endogenous YAP1. (C) WT- and Y188E-YAP1, but not Y188F-YAP1, restores cell proliferation. (***, p < 0.001) (D) Quantifications of shControl or shYAP1 alone, and shYAP1 plus WT- , Y188F- or Y188E-YAP1 transduced MDA-MB-231 cell migration. (***, p < 0.001) (E) WT-YAP1, but not Y188F-YAP1, induced 3D invasive structure in MDA-MB231 cells.
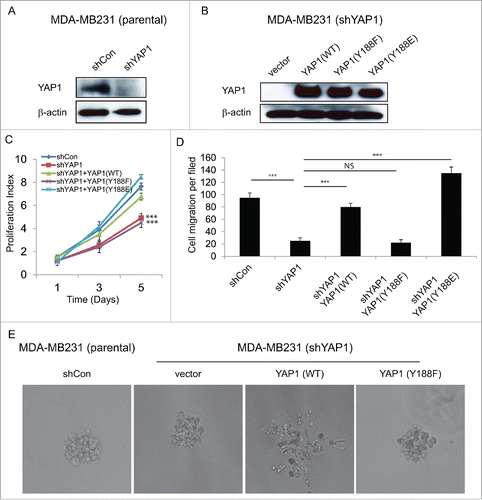
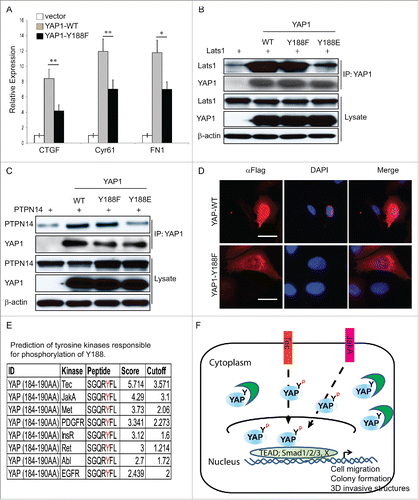
Figure 5. Disrupted interaction between YAP1-Y188E and its upstream negative regulators. (A) qRT-PCR analysis of the YAP1 target genes in MCF10A cells. GAPDH was used as an internal control. Error bars equal ±SD. (** p < 0.01; * p < 0.05) (B) Disrupted interaction between YAP1-Y188E and Lats1. HEK293T cells were transfected with LATS1 alone or with LATS1 plus WT- , Y188F- or Y188E-YAP. YAP1 was immunoprecipitated and the co-immunoprecipitated Lats1 was detected by immunoblot. (C) Disrupted interaction between YAP1-Y188E with PTPN14. HEK293T cells were transfected with PTPN14 alone or with PTPN14 plus WT- , Y188F- or Y188E-YAP1. YAP1 was immunoprecipitated and the co-immunoprecipitated PTPN14 was detected by immunoblot. (D) Overexpression of YAP1-Y188F induces YAP1 nuclear exclusion. Immunofluorescence microscopy shows cytoplasmic localization of YAP1-Y188F in transduced-293 cells, in contrast to the nuclear localization of YAP-WT. (scale bar = 20µm). (E) Prediction of tyrosine kinases responsible for the phosphorylation of Y188. (F) Working model for the phosphorylation modification of YAP1-Y188. The in silico predicted kinases phosphorylate YAP1-Y188, the YAP1-Y188 phosphorylation enhances YAP1 oncogenic function through YAP1 nuclear activation.