Figures & data
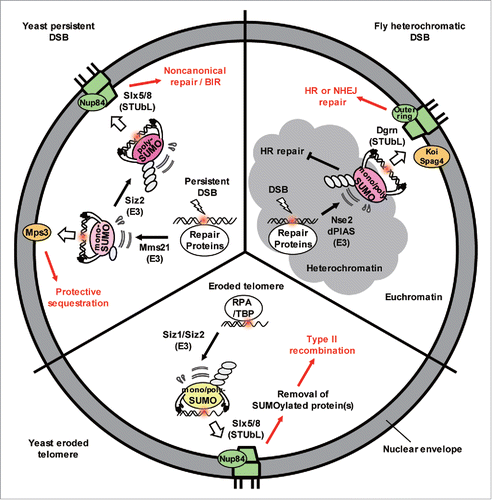
Figure 1. The extent of SUMO chain formation affects spatial sequestration of damage and the repair pathway choice. Repair proteins bind to DSBs and eroded telomeres in yeast and become modified by Mms21 and/or Siz1/Siz2 SUMO ligases. If monoSUMOylated, the DSBs shift to Mps3 where aberrant recombination is inhibited. If Siz2 adds a polySUMO chain, it is recognized by Slx5/Slx8, a STUbL enzyme that shifts the damage to nuclear pores. At the pore, ubiquitination of the polySUMOylated substrates and proteasome degradation facilitate alternative repair pathways. Similar events happen to DSBs in heterochromatin in flies (see text).