Figures & data
Figure 1. Diabetes inhibits AMPK and decreases mTORC1, Nrf2 and OGG1 protein expression. (A & C) Representative Immunoblot analysis shows a significant decrease in AMPK phosphorylation at Thr172 and raptor at Ser792 (marker of mTORC1 activation) in kidney cortex of db/db mice (d) compared to wild type mice (W). Inactivation of AMPK and activation of mTORC1 resulted in significant decrease of Nrf2 and OGG1 protein expression in kidney cortex of diabetic compared to wild type mice. Actin was used as a loading control. (B & D) Histograms represent means ± SE (n = 4). Significant difference from wild type is indicated by *P < 0.01.
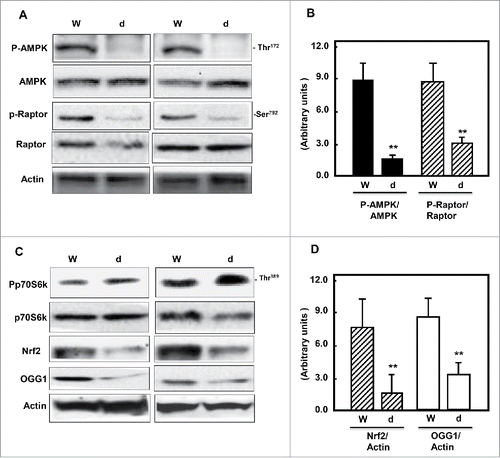
Table 1. AICAR improves kidney parameters in diabetic mice. Treatment of db/db mice with AICAR showed no significant changes in body weight, kidney weight and kidney weight/body weight compared to non-treated mice. On the other hand, significant decreased in hyperglycemia, proteinuria, albuminuria and urine creatinine were detected in AICAR treated mice compared to non-treated mice.
Figure 2. AICAR upregulates AMPK and inhibits mTORC1 to increase protein expression of Nrf2 and OGG1 and decrease 8-oxodG in kidney of diabetic mice. Immunoblot analysis shows that AICAR treatment increased phosphorylation of AMPK at Thr172 and resulted in significant decrease in mTORC1 measured by (A) increase in phosphorylation of raptor at Ser92 and (C) decrease in phosphorylation of p70S6k at Thr389 in kidney cortex of diabetic mice compared to non-treated mice. (C) Activation of AMPK resulted in significant increase in transcription factor Nrf2 and OGG1 protein expression in treated mice compared to non-treated mice. Actin was used as a loading control. (B&D) Histograms represent means ± SE (n = 4). (E) 8-OxodG levels were measured according to the instruction manual of 8-oxodG kits showed significant decrease in DNA damage in kidney of treated mice compared to non-treated mice. Significant difference from non-treated mice is indicated by **P < 0.01. Kidney sections of db/db mice (F) treated or non-treated with AICAR were stained with 8-oxodG antibody followed by FITC-anti-rabbit IgG as secondary antibodies. Staining of nucleus with propidium Iodide (PI) (red) and 8-oxodG with FITC (green) were detected using a filter with excitation range 450–490 nm and excitation at 535 nm.
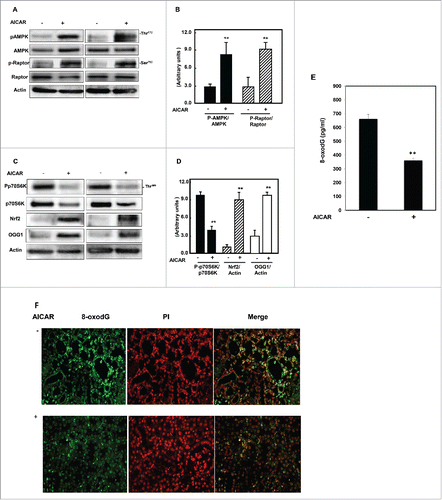
Figure 3. AICAR activates AMPK and decreases mTORC1 expression resulting in increased protein/mRNA expression and promoter activity of OGG1 and decreased 8-oxodG in HG-treated renal proximal tubular cells. High glucose significantly decreased AMPK phosphorylation and activates mTORC1 measured by decrease (A) in phosphorylation of raptor at Ser92 and (B) an increase in phosphorylation of p70S6k at Thr389 in MCT cells. (A) Cells treated with AICAR before exposure to HG show a significantly increased in activation of AMPK, which decreases mTORC1 expression. (B) Enhance AMPK activity and inhibition of mTORC1 by AICAR leads to upregulation of Nrf2 and OGG1 protein expression in cell exposed to HG. (C) RT-PCR of OGG1 was performed in RNA isolated from MCT cells grown in NG or HG and treated with AICAR. PCR products were analyzed on an ethidium bromide-stained gel. GAPDG was used a loading control. (D) AMPK activity was measured as phosphorylation levels of AMPK at Thr172 in HG or/and AICAR treated MCT cell lysates by AMPK ELISA kit. (E) A reporter plasmid containing the OGG1 promoter driving expression of the luciferase and a control Renilla reporter gene were co-transfected into the cells using LipofectAMINE Plus Reagent™. AICAR pretreatment reversed the inhibitory effect of HG on OGG1 promoter activity as well as significantly increased OGG1 activity in cells grown in NG. (F) DNA was extracted from treated and non-treated cells and digested with nuclease P1. The detection of dG and 8-oxodG was performed using 8-oxodG kits. Authentic standards of 8-oxodG were analyzed simultaneously. Experiment represent means±SE (n = 6). Significant difference from cells grown in normal glucose is indicated by *P < 0.01, cells grown in NG and treated with AICAR by **P < 0.01 and cells exposed to HG and treated with AICAR compared to cells grown in HG by #P < 0.01.
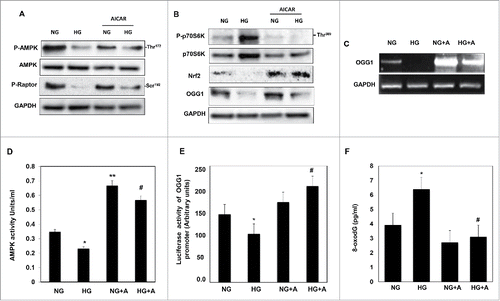
Figure 4. Downregulation of AMPK resulted in decreased Nrf2 and OGG1 protein expression and led to a significant decrease in OGG1 promoter activity in renal proximal tubular cells treated with HG. (A) Cells transfected with DN-AMPK resulted in downregulation of AMPK in cells under both conditions of NG and HG. Decrease in AMPK activity resulted in significant downregulation of Nrf2 and OGG1 protein compared to cells transfected with control (nonspecific siRNA). (B) Cells co-transfected with DN-AMPK and a reporter plasmid construct carrying the OGG1 promoter showed a significant decrease in OGG1 promoter activity in cells grown under low and high concentrations of glucose indicating the role of AMPK as a major kinase regulate DNA repair activity. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG is indicated by *P < 0.01, cells grown in NG and transfected with DN-AMPK by **P < 0.01 and cells exposed to HG and transfected with DN-AMPK compared to cells grown in HG transfected with control by #P < 0.01. Downregulation of raptor resulted in decreased AMPK activity, Nrf2 and OGG1 protein expression as well as OGG1 promoter activity in renal proximal tubular cells treated with HG. (C) Cells transfected with siRNA against raptor showed a decrease of AMPK activity in cells under both conditions of NG and HG. Decrease of raptor expression resulted in significant downregulation of Nrf2 and OGG1 protein compared to cells transfected with control (nonspecific siRNA). (D) Cells co-transfected with siRNA against raptor and a reporter plasmid construct carrying the OGG1 promoter showed a significant decrease in OGG1 promoter activity in cells grown under low and high concentrations. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG is indicated by *P < 0.01, cells grown in NG and transfected with siRNA of raptor by **P < 0.01 and cells exposed to HG and transfected with siRNA of raptor compared to cells grown in HG transfected with control (nonspecific siRNA) by #P < 0.01. Downregulation of Akt resulted in increase Nrf2 and OGG1 protein expression and significant increase in OGG1 promoter activity in renal proximal tubular cells treated with HG. (E) Cells transfected with DN-Akt showed a decrease Akt phosphorylation at Ser473 in cells under both conditions of NG and HG. Decrease in Akt activity resulted in significant upregulation of Nrf2 and OGG1 protein compared to cells transfected with control (nonspecific siRNA). (F) Cells co-transfected with DN-Akt and reporter plasmid construct carrying the OGG1 promoter showed a significant increase in OGG1 promoter activity in cells grown under NG and HG indicating the role of Akt as a major kinase in the regulation of the DNA repair activity. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG is indicated by *P < 0.01, cells grown in NG and transfected with DN-Akt by **P < 0.01 and cells exposed to HG and transfected with DN-Akt compared to cells grown in HG transfected with control (nonspecific siRNA) by #P < 0.01. Downregulation of rictor resulted in increase Nrf2 and OGG1 protein expression as well as OGG1 promoter activity in renal proximal tubular cells treated with HG. (G) Cells transfected with siRNA against rictor showed a significant decrease in rictor expression resulted in upregulation of Nrf2 and OGG1 protein compared to cells transfected with control (nonspecific siRNA). (H) Cells co-transfected with siRNA against rictor and plasmid construct carried OGG1 promoter showed a significant increase in OGG1 promoter activity in cells grown under low and high concentrations. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG is indicated by *P < 0.01, and cells exposed to HG and transfected with siRNA of rictor compared to cells grown in HG transfected with control (nonspecific siRNA) by #P < 0.01.
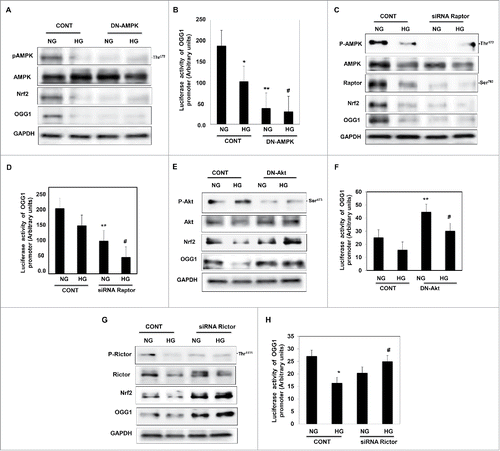
Figure 5. Downregulation of Nrf2 resulted in significant decrease in OGG1 protein expression and lead to sharp decrease in OGG1 promoter activity in renal roximal tubular cells treated with HG. (A) Cells transfected with siRNA against Nrf2 showed a significant decrease in OGG1 protein compared to cells transfected with control (nonspecific siRNA) and grown under both conditions of NG and HG. In addition, cells transfected with siRNA against Nrf2 and treated with AICAR showed no changes in protein expression of Nrf2 and OGG1. (B) Cells co-transfected with siRNA against Nrf2 and reporter plasmid construct carried OGG1 promoter showed a significant decrease in OGG1 promoter activity in cells grown under low and high concentrations. Cells transfected with siRNA against Nrf2 and treated with AICAR showed significant decrease in OGG1 promoter activity under both NG and HG conditions. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG compared to cells in HG is indicated by *P < 0.01, cells grown in NG and transfected with siRNA of Nrf2 by **P < 0.01, cells transfected with siRNA of Nrf2 and exposed to HG by #P < 0.01, cells grown in NG and transfected with siRNA of Nrf2+treated with AICAR by §P < 0.01, and cells transfected with siRNA of Nrf2 and exposed to HG+treated with AICAR by ¶P < 0.01.
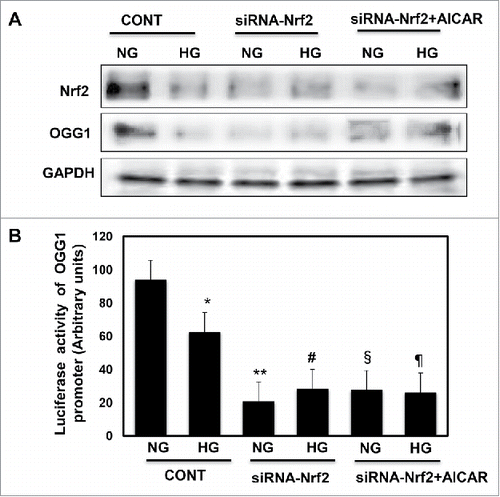
Figure 6. High glucose treatment significantly reduces binding of Nrf2 to the OGG1 promoter element and AICAR treatment reversed the effect of HG in renal proximal tubular cells. (A) EMSA analysis of a DNA probe corresponding to the putative Nrf2 binding site in the OGG1 promoter. Labeled probes were incubated with nuclear extracts isolated from MCT cells grown in NG or HG and treated or non-treated with AICAR (2 mM). (B) Treatment of MCT cells with HG significantly reduced binding of Nrf2 to OGG1 promoter compared to cells grown in NG. While AICAR treatment showed significant increased in binding of Nrf2 to OGG1 promoter activity in cells grown in NG and cells exposed to HG. (C) The specificity of binding of the DNA/protein complex to Nrf2 was demonstrated by adding an Nf2 antibody (1 and 2ug) to the reaction mixture. Including the Nrf2 antibody in the reaction results in marked reduction of the specific DNA/protein complex.
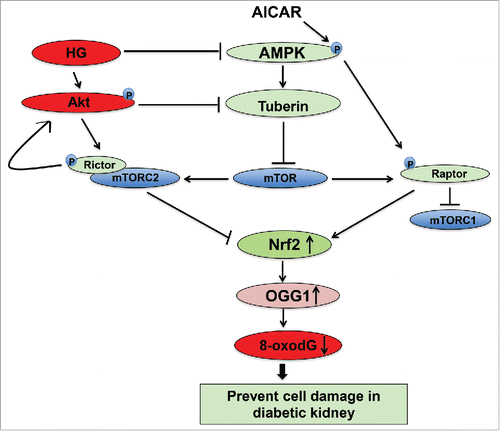
Figure 7. Proposed model for the role of AICAR in preventing kidney damage in diabetes. AICAR activates AMPK and inhibits binding of raptor to mTORC1 to decrease mTOR and increase OGG1 activity. Inactivation of AMPK results in downregulation Nrf2 and leads to decrease the functional activity of OGG1. On the other hand, inactivation of Akt and inhibition of mTORC2 results in upregulation of OGG1 activity to reduce accumulation of oxidative DNA damage in renal cells. The consequences steps of upregulation and downregulation of major signals that activate the DNA repair pathways and prevent cell damage lead to improved kidney parameters in diabetic patients under the therapeutic effect of AMPK activator.