Figures & data
Figure 1. Mitotic timing and the checkpoint response in HeLa cells remains largely unaffected by inducible expression of Cdk1AF (A) HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells were synchronised by a combined serum starvation and thymidine protocol (Materials and Methods), then washed and cultured in the presence (+DOX) or absence (-DOX) of doxycycline (DOX, 1 μg/ml). Cells at 0, 4, 8, 12 and 16 hours (h) post-release were collected for FACS and Western blotting. FACS profiles are shown in the left panel. Cdk1AF-Flag (FLAG), phosphorylated H3 (pH3) and Actin (Actin) at different time points detected by western immunoblotting are shown in the right panel. (B) HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells were synchronised as before. Immediately upon release (T=0), HeLa Tet-ON Cdk1AF cells were induced with DOX which remained in the media for the rest of the experiment. DNA damage was induced with Adriamycin (Adr: 300 nM) at 4 h (T = 4) post release. Aliquots of HeLa Tet-ON cells were concurrently treated with 5 mM caffeine (Caff) (T=4). To inhibit apoptosis, pan-caspase inhibitor Z-VAD was added to all samples at T=4. Cells were harvested for biochemical analysis or fixed for immunofluorescence at T=16. FACS profiles are shown in the left and middle panels. Levels of phospho-Chk1 (pChk1), Chk1, phospho-Chk2 (pChk2), Chk2 and Actin are shown in the right panel, with vertical boxes indicating protein levels at 8 h and 24 h. (C) Synchronised HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells were treated as described in . After 4 h exposure to Adriamycin (T=8), cells were stained with γH2AX (red) and DAPI (blue) (left panel). Inset shows mitotic cells, confirmed by the presence of biopolar spindles with β- tubulin (green) staining. For both γH2AX and Rad51 quantification: n> 500 for interphase; n > 200 for mitotic cells. Scale bar represents 5 μm. (D) Cells were collected at indicated time points and lysed. Histone H1 kinase activity was measured in immunoprecipitates obtained with anti-Cdk1 antibodies (to pull down Cdk1, left panel) or anti-FLAG (to pull down Cdk1-FLAG, right panel). An autoradiogram of Histone H1 with P-32 phosphate in the in vitro kinase assay is shown (Materials and Methods). Untreated cells (labeled as release) or cells treated with thymidine (Thy) or Nocodazol (Noc) were used as controls.
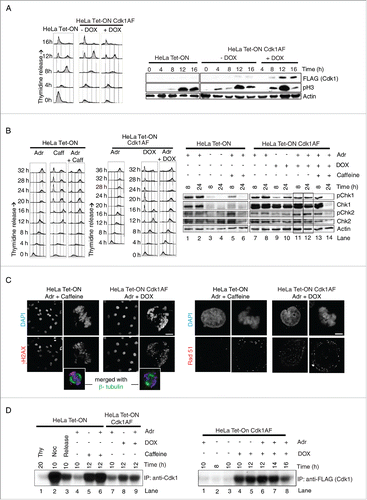
Figure 2. Mitotic entry and aberrant mitosis in damaged Cdk1AF cells. (A) HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells were synchronised as described in . After the release from thymidine arrest, HeLa Tet-ON Cdk1AF cells were treated with Adriamycin in the presence doxycycline (Adr+Dox), while HeLa Tet-ON cells were treated with Adriamycin (Adr) or Adriamycin and Caffeine (Adr+Caffeine) as controls. The cells were trapped in mitosis with nocodazole treatment as described in . Mitotic cells were harvested by shake-off at indicated time points from the same plates and were analyzed by FACS (Materials and Methods). The shake-off cell numbers were counted and cumulative mitotic cell numbers were plotted (left panel) as a ratio of mitotic cells to all viable cells collected (both floating and attached) by the end of experiment at T=24 h or T=45 h. Three independent experiments were performed. Samples from the same experiment were also subjected to MPM2-FACS analysis. Percentages of accumulative MPM2 positive cells are shown in the right panel. (B) Representative Immunofluorescence micrographs indicating mitotic hallmarks exhibited by HeLa Tet-ON Cdk1AF cells with DNA damage (synchronised and treated as described in ). After 4 h exposure to Adriamycin, damaged Cdk1AF cells began entering mitosis asynchronously. Immunofluorescence staining for mitotic spindles (β-tubulin, green), condensed chromosomes (pH3, red); cyclin B (red); lamin A/C (green) and Giantin (red) are shown. Scale bar represents 5 μm. (C) Synchronised HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells were treated as described in . HeLa Tet-ON cells treated with Adriamycin and caffeine (Adr+caffeine) or HeLa Tet-ON Cdk1AF cells treated with Adriamycin and doxycycline (Adr+DOX) were collected at T=16 and stained for DNA (DAPI, blue), kinetochores (ACA, green) and mitotic spindles (β-tubulin, red). Representative images are shown in the left panel. White arrows indicate prematurely decondensed chromosomes in metaphase, white arrowheads indicate chromosomes trapped in the cleavage furrow in late anaphase and telophase, and yellow arrows indicate fragmented chromatin in metaphase. The numbers (aberrant cells/total cells) indicate the frequency of occurrence of aberrant mitotic phenotypes in one experiment. Box plot (right panel) shows the quantification of aberrant mitotic figures. Cells in metaphase, anaphase and telophase/cytokinesis were scored according to chromosomal and kinetochore status/alignment/segregation, and collated. * indicates p<0 .005. Error bars represent 95% confidence intervals (CI). A total of 200 cells were counted for each condition in 3 independent experiments. (D) To inhibit ATR or Chk2, asynchronous HeLa Tet-ON Cdk1AF cells were transfected with siATR or siChk2 4 h prior to the synchronisation as described in respectively, then released and treated with Adr+DOX. To inhibit ATM, synchronised HeLa Tet-ON Cdk1AF cells were treated with KU-55933 4 h after release from thymidine. To inhibit Chk1, synchronised HeLa Tet-ON Cdk1AF cells were treated with PF477736 4 h after release from thymidine. Adr and DOX were added as described in . Cells were collected for vizualization and quantification of aberrant mitosis. Box plot shows rescuing of aberrant mitosis in damaged Cdk1AF cells by siRNA targeting ATR (siATR) and KU-55933 (ATM inhibitor). A total of 200 cells were counted for each condition in 3 independent experiments. * indicates p < 0 .005. Error bars represent 95% confidence intervals (CI).
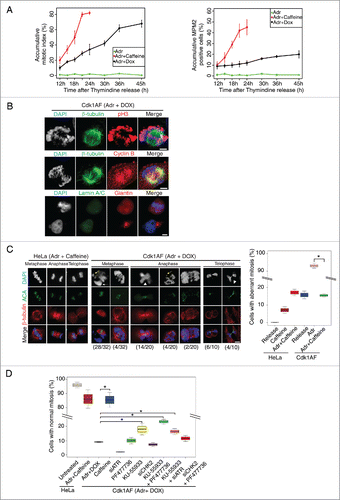
Figure 3. Loss of axial condensin localization in damaged Cdk1AF cells. (A) Synchronised HeLa Tet-ON, HeLa Tet-ON Cdk1AF cells clone B and clone D were treated as described in . As controls, damaged HeLa Tet-ON Cdk1AF cells clone B and clone D were also treated with Caffeine. These cells, trapped in mitosis using nocodazole, were harvested, hypotonically-swollen, fixed with ice cold methanol-acetone (1:1) and stained for the condensin subunit SMC2 (green), with chromosomes counterstained with DAPI. Representative images are shown. Scale bar represents 5 μm. Insert shows zoom-in of individual chromosomes. SMC2 intensity in the nuclei was quantitated using the threshold signal in DAPI staining to define the nuclear periphery (Materials and Methods). Box plots show the ratio of SMC2 to DAPI intensity (relative intensity) in the right panel. Error bars represent 95% confidence intervals (CI), n = 20, *indicates p < 0 .001; NS indicates no significant difference. (B) Synchronised HeLa Tet-ON, HeLa Tet-ON Cdk1AF cells clone B and clone D were treated as described in . Cells were collected and fixed with methanol: acetic acid (3:1) (Materials and Methods). Chromosome spreads were stained with SMC2 (red) with DNA mounted with DAPI (blue). Representative images are shown. Scale bar represents 5 μm. Insert shows zoom-in of individual chromosomes. (C) Synchronised HeLa Tet-ON, HeLa Tet-ON Cdk1AF clone D cells were treated as described in . Cells were stained for CAP-D2 (green), CAP-G2 (red) and DNA (DAPI). Representative images are shown. Scale bar represents 5 μm. Insert shows zoom-in of individual chromosomes. (D) Synchronised HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells were treated as described in and trapped in mitosis using nocodazole. Mitotic chromosomes from nocodazole-trapped cells were isolated and subjected to SDS-PAGE. The levels of SMC2, CAP-D2, CAP-G2, CAP-G, Histone H3 and ORC2 are shown in the left panel. Levels of SMC2, CAP-D2 and CAP-G2 in whole nuclear extracts (containing both nucleoplasmic and chromosomal materials) with the mitotic chromosomes from damaged HeLa Tet-ON cells treated with caffeine and damaged HeLa Tet-ON Cdk1AF cells treated with DOX are shown in the middle panel. Synchronised HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells were treated as described in , trapped with nocodazole and harvested by mitotic shake-off 12 h after adriamycin treatment. Whole cell lysates were prepared and immunoprecipitated (IP) with anti-CAP-D2, or anti-SMC2 or anti-CAP-G2 antibodies respectively. The immuno-complexes were resolved by SDS-PAGE and Western blotting were performed to detect SMC2, CAP-D2, and CAP-G2 respectively (right panel).
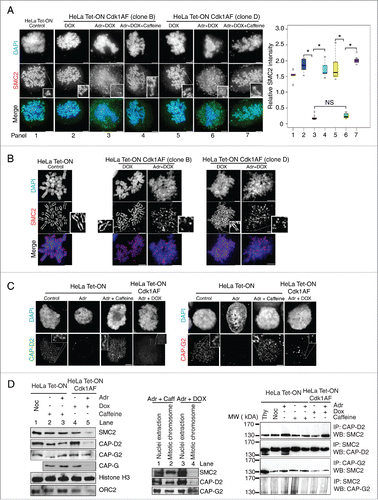
Figure 4. DNA damage response in U2OS Tet-ON Cdk1AF cells. (A) U2OS Tet-ON and U2OS Tet-ON Cdk1AF cells were treated in an identical manner to HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells as described in . Cells were collected at T=24 h. The state of pChk1, Chk1, pChk2 and Chk2 was monitored by Western blotting. (B) At T=16 h, U2OS Tet-ON Cdk1AF cells experienced an aberrant mitosis similar to that observed in HeLa Tet-ON Cdk1AF cells, whereas damaged U2OS Tet-ON cells treated with caffeine underwent normal mitosis (left panel). Cell were stained for mitotic spindle (β-tubulin, red), kinetochores (ACA, green) and DNA with (DAPI, blue). The numbers (aberrant cells/total cells) indicate the frequency of occurrence of aberrant mitotic phenotypes in one experiment. Representative images are shown. Scale bar represents 5 μm. (C) Synchronised U2OS Tet-ON, U2OS Tet-ON Cdk1AF cells were treated as described in . As controls, damaged U2OS Tet-ON Cdk1AF cells were also treated with Caffeine. These cells, trapped in mitosis using nocodazole, were harvested, hypotonically-swollen, fixed and stained for the condensin subunit SMC2 (red), with chromosomes counterstained with DAPI. As in the case of HeLa Tet-ON Cdk1AF cells, damaged U2OS Tet-ON Cdk1AF cells lose axial SMC2 localization which is retained in DNA-damaged U2OS Tet-ON cells treated with caffeine. Representative immunofluorescence staining images of anti- SMC2 (red) and counterstaining with DAPI are shown. Scale bar represents 5 μm.
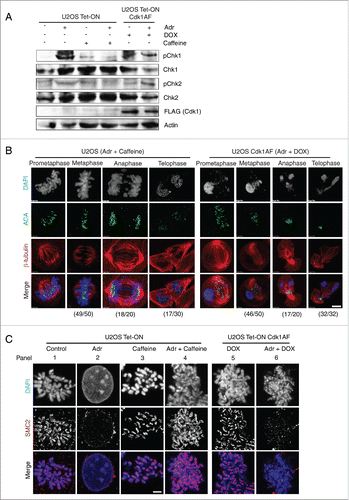
Figure 5. Inhibition of the ATM-Chk2 pathway restores SMC2 association with mitotic chromosomes. (A) Asynchronous HeLa Tet-ON Cdk1AF cells were transfected, respectively, with siRNA targeting Chk1, Chk2, ATR, and control siRNA (targeting a non-relevant gene), or mock transfected 24 h prior to synchronisation as described in . The efficacy of various siRNAs are shown (left panel). Cells were trapped in mitosis using nocodazole, fixed and stained with anti-SMC2 and DAPI. The histogram shows SMC2 axial localization in damaged HeLa Tet-ON Cdk1AF cells due to the knockdown of the targeted protein (central panel). Representative immunofluorescence micrographs illustrating different degrees of recruitment are shown in right panel. Scale bar represents 5 μm. Insert shows zoom-in of individual chromosomes. Three independent experiments were performed with similar results and data is shown for one experiment. 200∼400 cells were counted for each condition. Downregulation of respective proteins was confirmed by Western blotting analysis (left panel). Actin for siChk2 experiment was used as loading control. (B) Asynchronous HeLa Tet-ON Cdk1AF cells were transfected with siRNA targeting Chk2 24 h prior to synchronisation as described in , treated with or without specific inhibitor to Aurora B (ZM447439) 4 h after release from thymidine in the presence of Adr and DOX as described in . The histogram shows SMC2 axial localization in damaged HeLa Tet-ON Cdk1AF cells (right panel). Representative immunofluorescence micrographs illustrate that the Aurora B inhibitor (ZM447439) abrogates siRNA Chk2 mediated-recruitment of SMC2 localization (left panel). Scale bar represents 5 μm. Insert shows zoom-in of individual chromosomes.
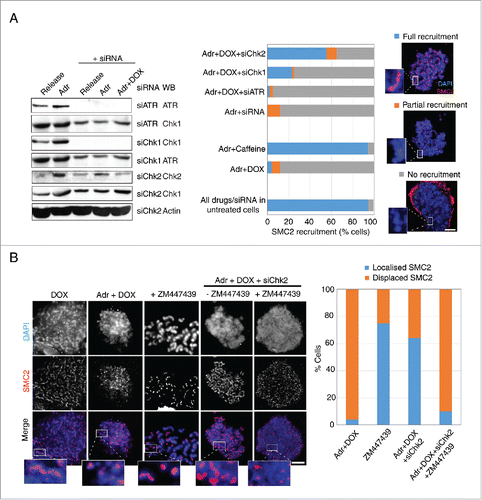
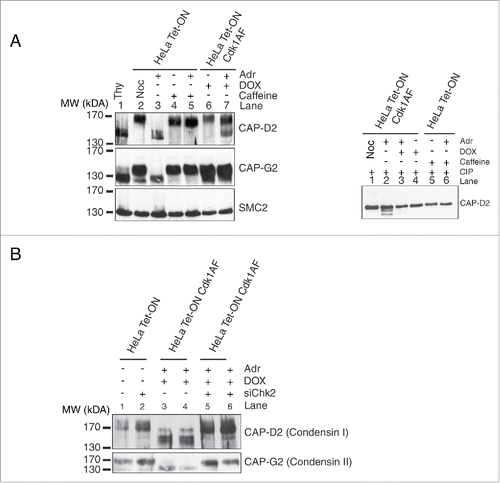
Figure 6. Post-translational modifications of condensin subunits induced by DNA damage. (A) Synchronised HeLa Tet-ON and HeLa Tet-ON Cdk1AF cells were treated as described in , trapped in mitosis using nocodazole and harvested by mitotic shake-off 16 h after Adriamycin treatment. Whole cell lysates were resolved on an 8% high cross-linking SDS-PAGE. Cells treated only with Adriamycin or nocodazole or thymidine were used as controls. The electrophoretic mobility shifts of CAP-D2, CAP-G2 and SMC2 are shown. The same lysates were treated with calf intestinal phosphatases (CIP) at 37°C for 20 minutes, and then subjected to high cross-linking SDS-PAGE, The mobility change of CAP-D2 is shown in the right panel. (B) Hela Tet-ON Cdk1AF cells transiently transfected with siChk2 were synchronised as described in , arrested with nocodazole and harvested by mitotic shake off. Western blotting was performed for the vizualization of the CAP-D2 and CAP-G2 bands.