Figures & data
Figure 1. Expression and localization of centriolin during mouse oocyte meiotic maturation. A. mRNA expression of centriolin during oocyte meiotic maturation. Each sample contained 50 oocytes. B. mRNA levels for centriolin during mouse oocyte meiosis. The mRNA levels of centriolin at the Pro-MI, MI, and MII stages were 117 ± 33%, 107 ± 18%, and 314 ± 67%, respectively. C. Centriolin localization during oocyte meiotic maturation. During meiosis I, it was distributed on the spindles, and concentrated on the spindle poles at metaphase, but then relocated to the midbody at telophase. Green, centriolin; red: DNA. Bar = 20 μm.
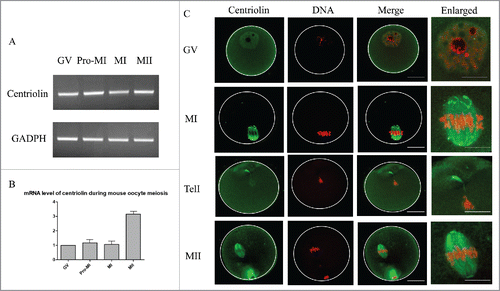
Figure 2. Distribution of centriolin after treatment of metaphase oocytes with nocodazole. After disruption of the meiotic spindle, centriolin disappeared from the spindle and spindle poles. At least 30 oocytes were observed in 3 replicates. Red, centriolin; green, microtubules; blue, DNA. Bar = 20 μm.
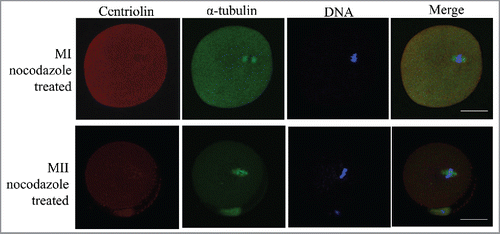
Figure 3. Knock-down of centriolin by Morpholino (MO) microinjection did not affect meiotic oocyte maturation process, but disrupted asymmetric division. A, B: Centriolin was effectively knocked down after microinjection a centriolin-specific MO. A total of 200 oocytes were used in each group. C: Depletion of centriolin did not affect completion of meiosis I. The polar body emission rate in the centriolin MO-injected group was the same as that in the control MO group after 10 -12 h of culture. A total of 166 oocytes were assessed in the MO group and 154 oocytes were assessed in the control group. D, E: A significantly higher proportion of oocytes underwent symmetric division after depletion of centriolin (p < 0.05).
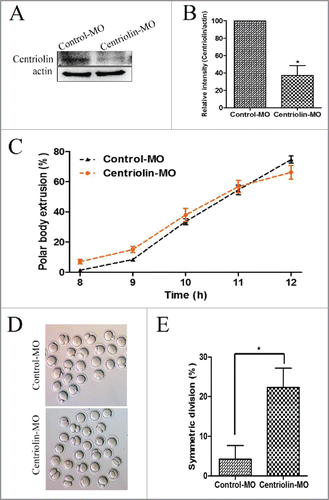
Figure 4. Knock-down of centriolin by siRNA microinjection did not affect meiotic oocyte maturation process, but led to the production of equal-sized cells or a large polar body with normal MII spindles. A, B: Effective depletion of centriolin after microinjection of siRNA into the oocyte. A total of 200 oocytes were used in centriolin siRNA-injected group, and a total of 200 oocytes were used in the control group. C: Similar percentages of mature oocytes were observed in siRNA group and control siRNA groups. At least 100 oocytes were assessed in each replicate. D. Big polar rate and symmetric division rate after centriolin siRNA knock-down. E. Centriolin knock-down produce equal-sized cells or a large polar body with normal MII spindles.
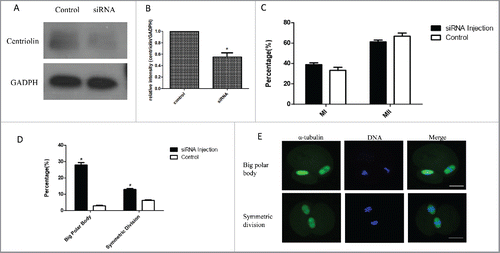
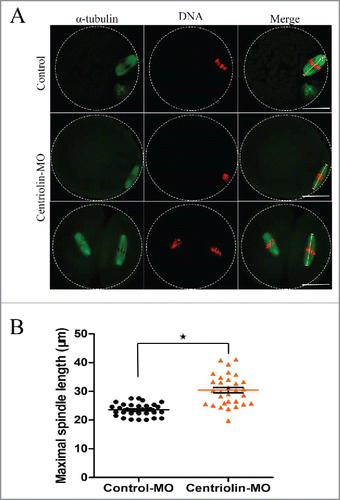
Figure 5. Centriolin depletion by Morpholino (MO) injection affects spindle size and peripheral spindle migration in oocytes. A: In control oocytes, most spindles moved to the cortex at late MI, whereas in centriolin MO-injected oocytes, spindles were centrally located. Bar = 20 μm B: The distance between the spindle and plasma membrane in the control MO-injected group was significantly shorter than that in the centriolin MO-injected group (p < 0.05). C: The length of the MI spindles in the centriolin MO-injected group was significantly longer than that of spindles in the control MO-injected group.
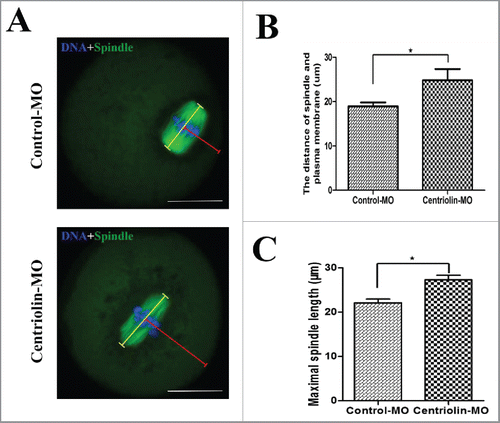
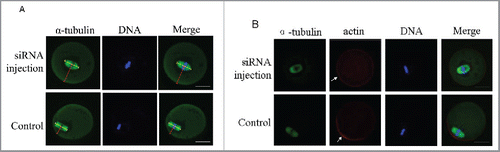
Figure 6. Centriolin siRNA microinjection inhibited peripheral spindle localization and polarity establishment in oocytes. A: Centrally localized MI spindles in centriolin depleted oocytes. At least 100 oocytes were assessed in each replicate. B: Centriolin siRNA microinjection led to a failure of polarity establishment, as indicated by the loss of an actin-cap. At least 50 oocytes were assessed in each replicate. Green, spindle; blue, DNA; red, actin. Bar = 20 μm.