Figures & data
Figure 1. Characterization of polyclonal antibodies that recognize Wee1 and Cdc25. (A) An anti-Cdc25 antibody was used to probe samples from wild type and cdc25Δ cdc2–3w cells. (B) An anti-Wee1 antibody was used to probe samples from wild type, wee1–3xHA, and wee1Δ cells. The HA tag causes a shift in the electrophoretic mobility of bands corresponding to Wee1. (C) Western blots of log phase Cdc25 and Wee1 with or without treatment with λ-phosphatase. Asterisks indicate background bands that serve as loading controls.
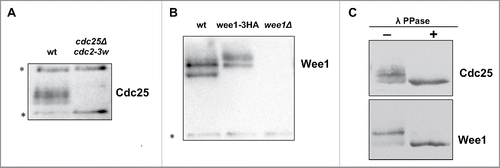
Figure 2. Cell cycle-dependent changes in phosphorylation of Wee1 and Cdc25. Fission yeast cells were synchronized by centrifugal elutriation and released into fresh medium at 30°C. The data in panels A and B were generated from the same time course to allow direct comparison of the timing of cell cycle events. (A) Western blots showing the behavior of Wee1, Cdc25, Cdc13, and Cdk1 inhibitory phosphorylation during the cell cycle. A background band was used as a loading control. A single asterisk is used to mark a partially phosphorylated form of Wee1; 2 asterisks mark more extensively phosphorylated forms referred to in the text as hyperphosphorylated forms. Inhibitory phosphorylation of Cdk1 was detected using a phospho-specific antibody. (B) A fluorescence microscopy assay using DAPI and calcofluor staining was used to determine the percentage of binucleated cells and cells undergoing septation.
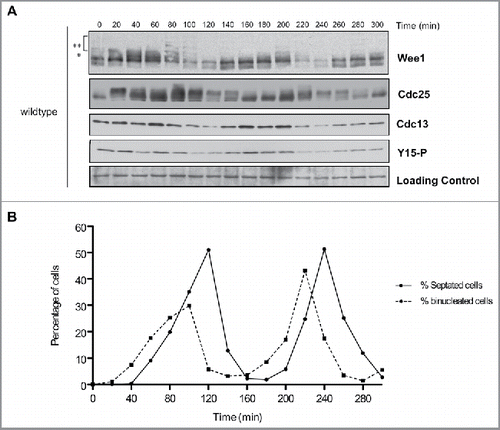
Figure 3. Cell cycle-dependent changes in phosphorylation of Cdc25 are dependent upon Cdk1 consensus sites. (A) Cdc25 was analyzed by western blot in asynchronous wild type, cdc25-13A and cdc25Δ cdc2-3w cells. (B) Cdc25, Cdc13, and Cdk1 inhibitory phosphorylation were analyzed by western blot in cdc25–13A cells synchronized by centrifugal elutriation. a-tubulin was used as a loading control. (C) A fluorescence microscopy assay using DAPI and calcofluor staining was used to determine the percentage of binucleated cells and cells undergoing septation.
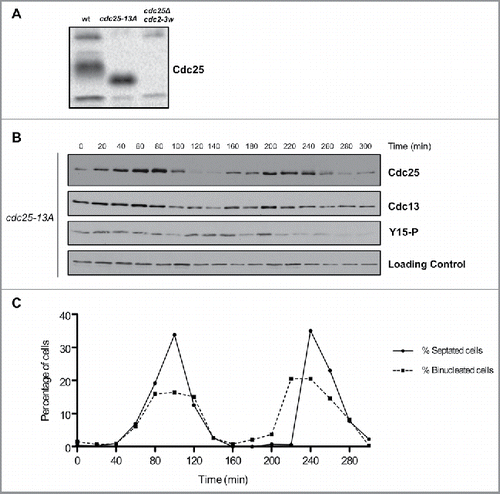
Figure 4. PP2APab1 is required for cell-cycle dependent changes in phosphorylation of Wee1 and Cdc25. (A) Wee1, Cdc25, Cdc13, and Cdk1 inhibitory phosphorylation were analyzed by western blot in pab1Δ cells synchronized by centrifugal elutriation, as in . A background band was used as a loading control. (B) A fluorescence microscopy assay using DAPI and calcofluor staining was used to determine the percentage of binucleated cells and cells undergoing septation.
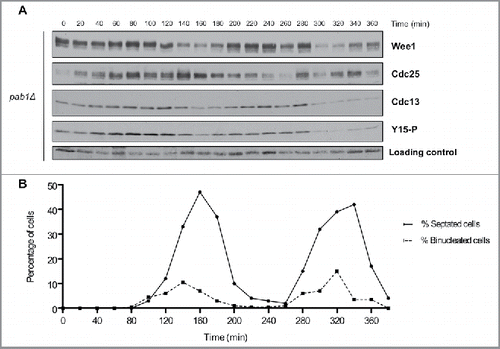
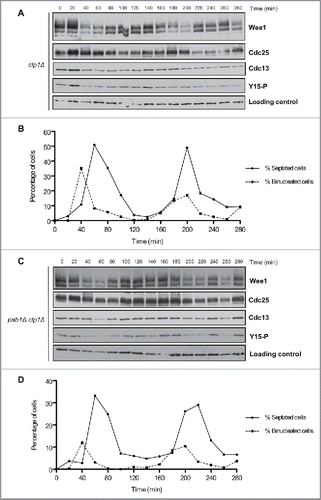
Figure 5. Inactivation of Clp1 and PP2APab1 does not show additive effects upon phosphorylation of Wee1 or Cdc25. (A, C) Western blots of Wee1, Cdc25, Cdc13, and Cdk1 inhibitory phosphorylation during the cell cycle in clp1Δ and pab1Δ clp1Δ cells synchronized by centrifugal elutriation. A background band was used as a loading control. (B, D) A fluorescence microscopy assay using DAPI and calcofluor was used to determine the percentage of binucleated cells and cells undergoing septation.