Figures & data
Figure 1. Confirmation of TSPO expression in Jurkat cells after stable transfection of TSPO expressing plasmids. (A) Relative abundance of exogenous TSPO mRNA expression in genetically modified Jurkat cell lines. Bar graph shows exogenous TSPO mRNA levels normalized to GAPDH and β-actin mRNAs. Expression of endogenous TSPO mRNA in MDA-MB-231 cells was set to 1.0. Jurkat cells transfected with TSPO expressed a comparable level of TSPO mRNA (exogenous) with that of the MDA-MB-231 cells (endogenous). No exogenous TSPO mRNA could be detected in wild-type and empty plasmid Jurkat cells. The data are presented as means ± SD (n = 4). (B) The level of TSPO protein expression in Jurkat cells was measured by radioligand binding using the TSPO specific ligand [3H]PK11195. The absence of specific binding in the wild-type and empty plasmid Jurkat cells confirms the negiligible level of TSPO protein. TSPO-Jurkat cells exhibited specific [3H]PK11195 binding though its Bmax is about one-third of that obtained from the positive control MDA-MB-231 cells. (C) Endogenous and exogenous expression of TSPO in Jurkat cells was determined by immunostaining with a TSPO specific antibody (green). There was no positive immunostaining observed in both wild-type and empty plasmid transfected Jurkat cells. However, strong staining with localized intracellular distribution along with mitochondria (red) was observed in TSPO-Jurkat cells. The blue color indicates the staining of nuclei. The yellow fluorescence in the right image demonstrates the overlap between TSPO and mitochondria. Scale bars: 10 μm.
![Figure 1. Confirmation of TSPO expression in Jurkat cells after stable transfection of TSPO expressing plasmids. (A) Relative abundance of exogenous TSPO mRNA expression in genetically modified Jurkat cell lines. Bar graph shows exogenous TSPO mRNA levels normalized to GAPDH and β-actin mRNAs. Expression of endogenous TSPO mRNA in MDA-MB-231 cells was set to 1.0. Jurkat cells transfected with TSPO expressed a comparable level of TSPO mRNA (exogenous) with that of the MDA-MB-231 cells (endogenous). No exogenous TSPO mRNA could be detected in wild-type and empty plasmid Jurkat cells. The data are presented as means ± SD (n = 4). (B) The level of TSPO protein expression in Jurkat cells was measured by radioligand binding using the TSPO specific ligand [3H]PK11195. The absence of specific binding in the wild-type and empty plasmid Jurkat cells confirms the negiligible level of TSPO protein. TSPO-Jurkat cells exhibited specific [3H]PK11195 binding though its Bmax is about one-third of that obtained from the positive control MDA-MB-231 cells. (C) Endogenous and exogenous expression of TSPO in Jurkat cells was determined by immunostaining with a TSPO specific antibody (green). There was no positive immunostaining observed in both wild-type and empty plasmid transfected Jurkat cells. However, strong staining with localized intracellular distribution along with mitochondria (red) was observed in TSPO-Jurkat cells. The blue color indicates the staining of nuclei. The yellow fluorescence in the right image demonstrates the overlap between TSPO and mitochondria. Scale bars: 10 μm.](/cms/asset/7c4cd746-8b95-4ed6-b842-5eaba8bd1353/kccy_a_1281477_f0001_oc.gif)
Figure 2. Changes in mRNA abundance following TSPO transfection. (A) Microarray analysis was used to detect differences in gene expression between the 3 Jurkat cell types. GSEA results comparing empty plasmid and TSPO-Jurkat cells were visualised using Enrichment Map Cytoscape Plug-in. Gene sets are represented by circular nodes that are proportional to the number of genes present in the gene set. The thickness of the lines between nodes is proportional to the overlap between gene sets. Nodes with highly similar gene sets are placed close to each other to form clusters. Red nodes are upregulated and blue nodes are downregulated. Expanded clusters in high resolution are shown in Figs. S1–S6. (B) Heat map of the leading edge genes from the mitochondrial electron transport chain cluster. The top 60 genes are shown with high expression represented in red. (C) Gene expression of mitochondrial respiratory chain complex IV (COX IV, subunit 2) in the TSPO-Jurkat cells was decreased by the TSPO ligand PK11195 (100 nM) when compared with control (without PK11195 but containing 0.1% DMSO). The same concentration of PK11195 had no effect on the wild-type and empty plasmid Jurkat cells.
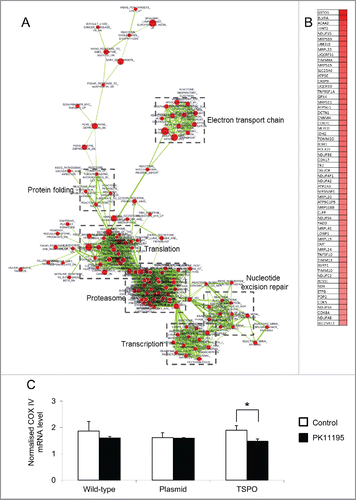
Figure 3. TSPO increased mitochondrial ATP production. (A) Averaged time-course of ATP production from permeabilised Jurkat cells was measured with luciferin-luciferase and a microplate reader. ATP production after injection of 2 mM ADP was markedly increased in all 3 cell types (wild-type, empty plasmid and TSPO-Jurkat cells). ATP production in TSPO-Jurkat cells increased at a greater rate than that in wild-type and empty plasmid Jurkat cells. (B) Accumulated ATP following injection of ADP in TSPO-Jurkat cells, calculated by subtracting the baseline (before ADP injection), was significantly greater than that observed in wild-type and empty plasmid Jurkat cells.
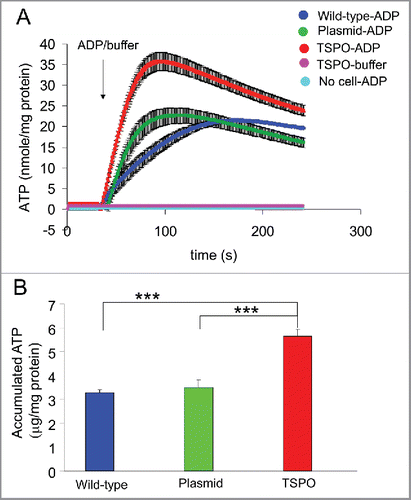
Figure 4. TSPO increased cell proliferation and motility. (A) Time course of Jurkat cell proliferation normalized to day 0, i.e., 1 hour after plating each of the cell types. TSPO-Jurkat cells proliferated significantly faster than wild-type and empty plasmid Jurkat cells at all time points measured (i.e. days 1–3). There was no difference in proliferation rate between wild-type and empty plasmid Jurkat cells. (B) TSPO-Jurkat cells had significantly higher spontaneous movement/migration compared with wild-type and empty plasmid Jurkat cells measured with the Boyden chamber (Transwell). The inhibitory effect of TSPO specific ligand PK11195 on spontaneous movement/migration was only seen in the TSPO-Jurkat cells, indicating the effect is TSPO-specific. (C) Foetal bovine serum (FBS, 0.1%) in DMEM, a chemo-attractant, non-specifically attracted all 3 types of Jurkat cells. However, PK11195 at 100 nM significantly decreased FBS-induced cell migration in TSPO-Jurkat cells only.
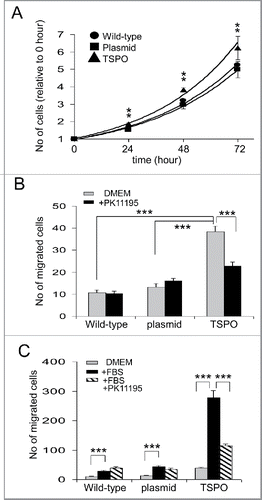
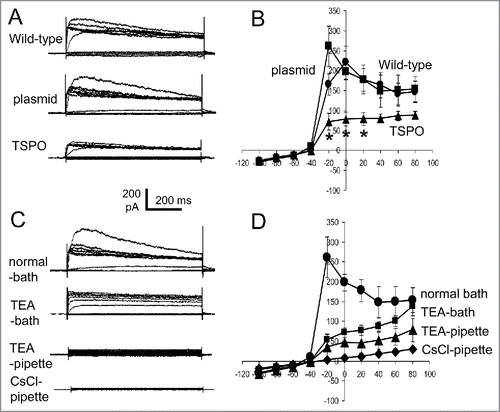
Figure 5. TSPO decreased whole-cell outward rectified K+ currents. (A) Episodic current traces of voltage-activated (from −100 mV to +80 mV with 20 mV increment for A, C) outward currents recorded in wild-type, empty plasmid, and TSPO-Jurkat cells. The membrane potential was held at −60 mV. The shape and amplitude of voltage induced outward currents in wild-type and empty plasmid Jurkat cells were comparable, while the amplitude in TSPO-Jurkat cells was smaller. (B) Current-voltage (I-V) curves of voltage-activated currents shown in (A). The amplitude of voltage-gated outward currents in TSPO-Jurkat cells was significantly smaller at −20 mV to 20 mV than either wild-type or empty plasmid Jurkat cells. (C) Current traces of empty plasmid Jurkat cells in normal bath solution (containing NaCl) and normal pipette solution (containing KCl), in normal bath solution with addition of 20 mM TEA-Cl, in normal pipette solution with addition of 20 mM TEA-Cl, and in a pipette solution with CsCl replacing KCl. TEA in the bath solution partly inhibited the voltage-gated outward current, but a greater effect was seen when TEA was in the pipette solution. Cs+ replacement of K+ in the pipette solution abolished the outward currents. (D) The I-V curves of corresponding traces shown in (C).