Figures & data
Figure 1. Characterization of hUC-MSCs and determination of cell viability after infection with T. gondii. (A) The expression levels of MSC-specific human CD markers were analyzed by flow cytometry. (B) Cell viability was measured by the MTS assays (means ± SD). * P < 0.05, ** P < 0.01 as compared with uninfected control cells. (C) The morphology of hUC-MSCs infected with T. gondii at the indicated time points. Scale bar = 100 μm.
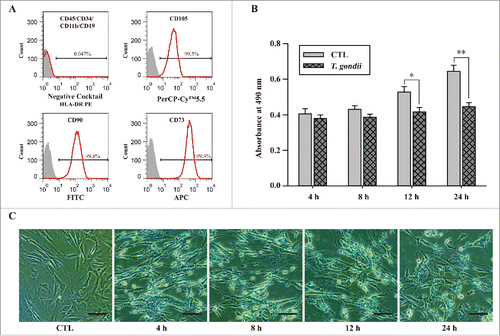
Figure 2. T. gondii infection rates and the expression levels of autophagy-related proteins in hUC-MSCs. (A) hUC-MSCs were infected with GFP-expressing T. gondii at an MOI of 5 for the indicated time durations. Cells were fixed and stained with Texas Red-X phalloidin to label F-actin (red), and nuclei were stained with DAPI (blue). Scale bar = 100 μm. (B) The number of T. gondii-infected cells and the total number of cells were counted to calculate the infection rate. *P < 0.05, **P < 0.01 as compared with cells infected with T. gondii for 4 h. (C) Cell lysates were subjected to western blotting using autophagy-related antibodies (LC3B and p62) and a T. gondii-specific antibody (TP3). (D) The expression levels of p-mTOR, mTOR and its downstream effectors S6K1 (phosphorylation at Thr389) and 4E-BP1 (phosphorylation at Thr37/46) were detected by western blotting. All data shown are representative of 3 independent experiments with similar results.
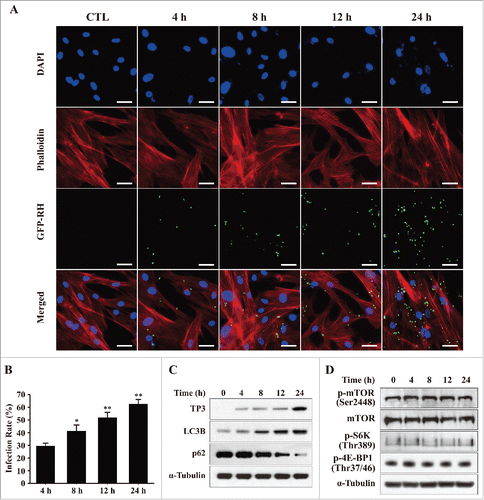
Figure 3. T. gondii-induced Mcl−1 downregulation and apoptosis in hUC-MSCs. hUC-MSCs were infected with T. gondii at an MOI of 5 for the indicated time durations. (A) Left, the protein levels of selected Bcl−2 family members were quantified by western blotting with α-Tubulin as a loading control. Representative blots of 3 independent experiments with similar results are shown. Right, fold changes of Mcl−1 levels in comparison with the results for the cells without T. gondii infection after normalization with the α-Tubulin level are shown (**P < 0.01). (B) The expression levels of PARP and cleaved caspase-3 in hUC-MSCs were determined by using western blotting. Three independent experiments were conducted showing similar results, and one representative blot is shown.
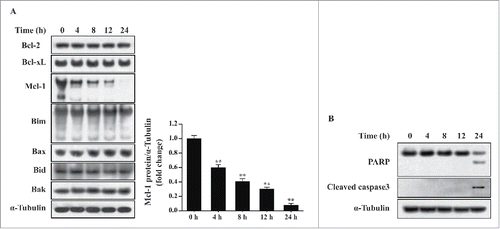
Figure 4. Overexpression of Mcl−1 inhibited T. gondii-induced autophagy and apoptosis in hUC-MSCs. (A) The Mcl−1 mRNA levels in hUC-MSCs were determined by qRT-PCR after adenoviral infection. * P < 0.05, ** P < 0.01 as compared with Ad/DsRed control groups. (B) The protein levels of Mcl−1 after T. gondii infection were determined by western blotting. (C) hUC-MSCs overexpressing Mcl−1 were infected with T. gondii at an MOI of 5 for 24 h, and the protein levels of Mcl−1 as well as endogenous LC3B, p62 and cleaved caspase-3 protein levels were quantified by western blotting. α-Tubulin was used as a loading control. All data shown are representative of 3 independent experiments with similar results.
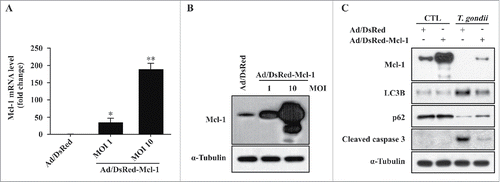
Figure 5. Ultrastructural morphology of normal and T. gondii-infected hUC-MSCs overexpressing Mcl−1. The ultrastructural morphology of hUC-MSCs infected with adenovirus expressing (A) DsRed alone or (B) DsRed-Mcl−1. hUC-MSCs overexpressing (C) DsRed and (D) DsRed-Mcl−1 were infected with T. gondii at an MOI of 5 for 24 h, and autolysosomes (red arrows) were detected by using TEM. hUC-MSCs overexpressing (E) DsRed and (F) DsRed-Mcl−1 were infected with T. gondii at an MOI of 5 for 24 h, and autophagosomes (red asterisks) were detected by TEM.
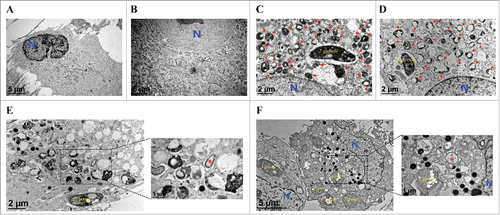
Figure 6. Mcl−1 interacted with Beclin-1 in the mitochondria. (A) hUC-MSCs were infected with T. gondii at an MOI of 5 for 24 h, and the cells were fractionated into the HM (containing mitochondria) and the LM (containing ER). Fractions were analyzed for the expression of Mcl−1, Bcl−2, Bcl−xL, Beclin-1, COX IV (a hallmark of mitochondria) and Calreticulin (a hallmark of ER). (B) Mcl−1 was immunoprecipitated from the HM and LM, and the expression of Mcl−1 and Beclin-1 were analyzed by western blotting. All data shown are representative of 3 independent experiments with similar results.
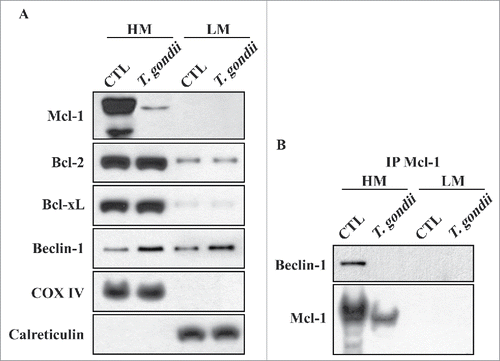
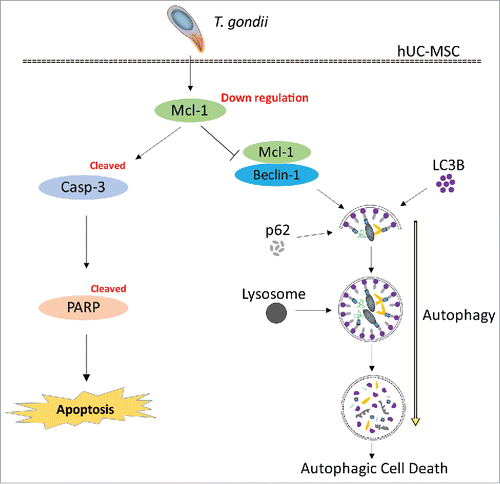
Figure 7. Suggested pathway of Mcl−1-controlled autophagy and apoptosis in hUC-MSCs infected with T. gondii. During the early infection stage (within 8 h), T. gondii reduces the protein levels of Mcl−1 in the mitochondria, which is followed by interruption of the Mcl−1/Beclin-1 complex. Consequently, there is accelerated formation of autolysosomes and autophagosomes. During the late stages of infection (up to 24 h), T. gondii down-regulates the protein levels of Mcl−1 in hUC-MSCs and activates caspase-3, which leads to PARP cleavage and the eventual induction of apoptosis.