Figures & data
Figure 1. Subcellular localization of Smc1β during mouse oocyte meiotic maturation. Mouse oocytes were microinjected with Smc1β-GFP cRNA at GV stage, and then cultured to GVBD, Pro-MI, MI, ATI and MII stages, respectively, followed by nuclear staining with Hoechst (blue). GV, oocytes at germinal vesicle stage; GVBD, oocytes at germinal vesicle breakdown stage; Pro-MI, oocytes at first prometaphase stage; MI, oocytes at first metaphase stage; ATI, oocytes at first anaphase/telophase stage; MII, oocytes at second metaphase stage. Scale bar, 20 μm.
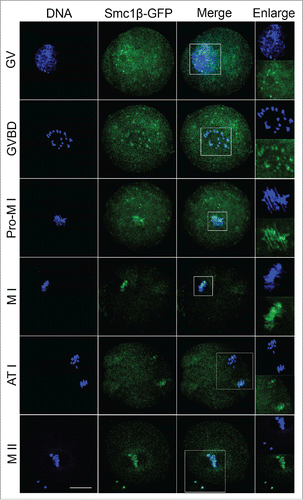
Figure 2. Knockdown of Smc1β causes spindle/chromosome abnormalities in mouse oocytes. (A) Protein levels of Smc1β in control and Smc1β-MO (morpholino injected) oocytes. The blots were probed with anti-Smc1β antibody and anti-GAPDH antibody, respectively. (B) Representative images of spindle morphologies and chromosome alignment in control and Smc1β-MO oocytes. Oocytes were immunostained with anti-α-tubulin-FITC antibody to visualize spindles and counterstained with Hoechst to visualize chromosomes. Scale bar, 20μm. (C) The proportion of abnormal spindles was recorded in control and Smc1β-MO oocytes. Data were presented as mean percentage (mean ± SEM) of at least 3 independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance. (D) The proportion of misaligned chromosomes was recorded in control and Smc1β-MO oocytes. Data were presented as mean percentage (mean ± SEM) of at least 3 independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.
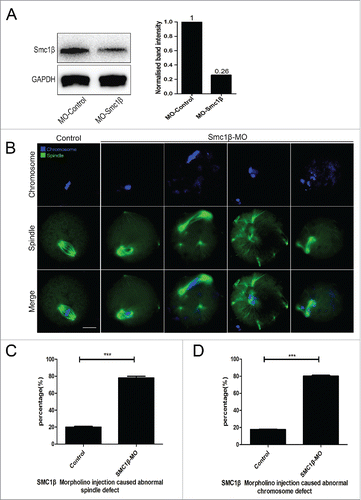
Figure 3. Knockdown of Smc1β leads to the disrupted kinetochore-microtubule attachment in mouse oocytes. (A) Representative images of kinetochore-microtubule attachments in control and Smc1β-MO oocytes. Oocytes were immunostained with anti-α-tubulin-FITC antibody to visualize spindles, with CREST to visualize kinetochores, and counterstained with Hoechst to visualize chromosomes. Scale bar, 5 μm. (B) The proportion of defective kinetochore-microtubule attachments was recorded in control and Smc1β-MO oocytes. Data were presented as mean percentage (mean ± SEM) of at least 3 independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.
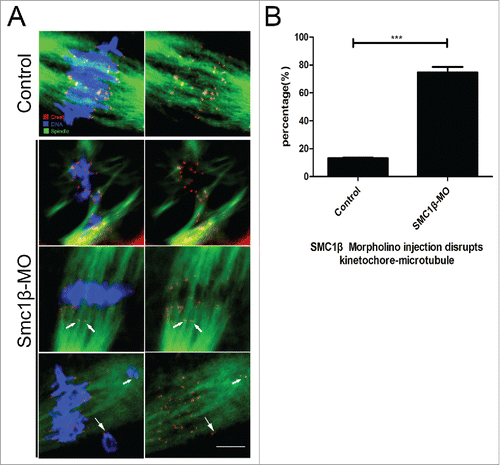
Figure 4. Knockdown of Smc1β results in the generation of aneuploidy in mouse eggs. (A) Representative images of euploid and aneuploid MII eggs. Chromosome spread was performed to calculate the number of chromosomes. Chromosomes were counterstained with PtdIns. Scale bar, 5 μm. (B) The proportion of aneuploid eggs was recorded in control and Smc1β-MO oocytes. Data were presented as mean percentage (mean ± SEM) of at least 3 independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.
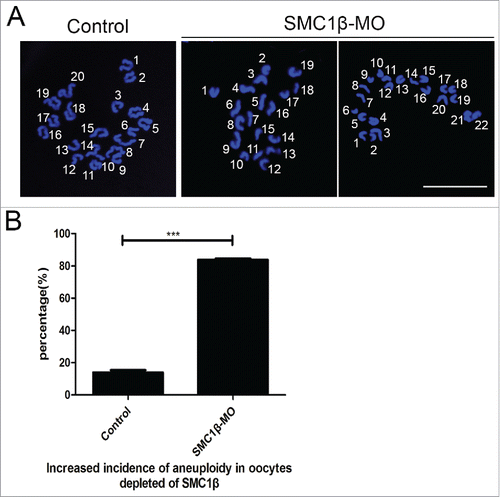
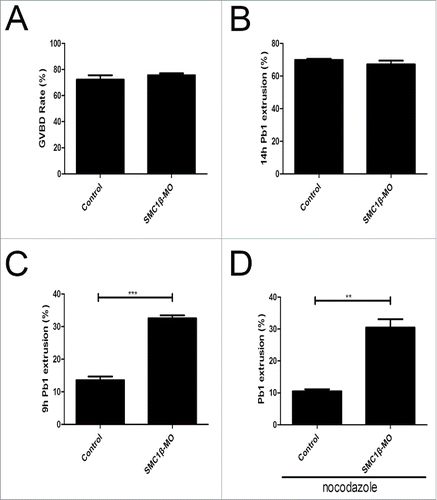
Figure 5. Effects of Smc1β Knockdown on the meiotic progression and SAC activity in mouse oocytes. (A) The proportion of germinal vesicle breakdown was recorded in control and Smc1β-MO oocytes. (B) The proportion of polar body extrusion was recorded in control and Smc1β-MO oocytes at the time point of 12 h post-GVBD. (C) The proportion of precocious polar body extrusion was recorded in control and Smc1β-MO oocytes at the time point of 7 h post-GVBD. (D) The proportion of overriding MI arrest was recorded in control and Smc1β-MO oocytes by low dose of nocodazole treatment. Data were presented as mean percentage (mean ± SEM) of at least 3 independent experiments. Asterisk denotes statistical difference at a p < 0.05 level of significance.