Figures & data
Figure 1. Localization of vinexin at the midbody during mitosis. (A) Live imaging of asynchronized HeLa cells overexpressing EGFP-vinexin β (Vxnβ) and RFP-Histone 2B (H2B). Midbody dense structure formed during cytokinesis observed by differential interference contrast (DIC) microscopy. Arrows and arrowhead mark midbody and focal adhesion, respectively. (B) After nocodazole release, endogenous vinexin was distributed in the cytosol from metaphase to anaphase but enriched at the midbody during cytokinesis. The mitotic stages were defined by the patterns of α-tubulin and Hoechst staining. (B′) A 3D-image reconstructed from confocal z stacks to show the entire midbody structure. (C) Isolated midbodies from Chinese hamster ovary (CHO) cells immunostained for vinexin and α-tubulin. Scales, 10 µm.
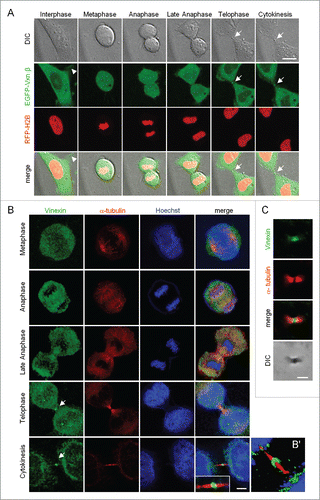
Figure 2. Depletion of vinexin reduces cell proliferation by delaying mitotic progression. (A) HeLa cells infected with a lentivirus expressing control (siCtrl) or shRNA against human vinexin (siVxn-1 or siVxn2) were harvested for immunoblotting assay of vinexin β and α-tubulin. (B) The proliferation rates of siCtrl and siVxn cells were monitored daily by Prestoblue assay. (C) Double thymidine-arrested siCtrl and siVxn cells were released to fresh medium for 12 h, then underwent α-tubulin immunostaining and Hoechst labeling. The midbody stage is when 2 daughter cells are still connected by the intercellular bridge, which is detected by α-tubulin immunostaining (arrows, a magnified example image is shown). Scale, 50 μm. The distribution of siCtrl or siVxn cells at the midbody stage and interphase expressed as mean ± SEM relative ratios with the ratio in siCtrl cells arbitrarily set to 1. Total cell number for each siRNA group from 3 independent experiments is > 1000. ** P < 0.01, * P < 0.05 by Student t test.
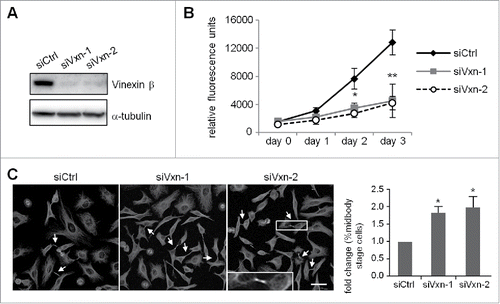
Figure 3. Knockdown of vinexin delays cell abscission. (A) SiCtrl and siVxn HeLa cells were synchronized at G2/M by nocodazole, then released for the indicated time before flow cytometry. Asynchronized siCtrl and siVxn cells were also included. The proportion of siCtrl and siVxn cells at G2 are expressed as mean ± SEM from 3 independent experiments. ** P < 0.01 comparing siCtrl and siVxn groups at each time by Student t test. (B) Time-lapse comparison of cytokinesis in a representative siCtrl cell (top) and 2 siVxn cells (middle and bottom). G1/S synchronized siCtrl and siVxn cells after release from the thymidine block. Midbodies remained at the center of intercellular bridge and midbody remnants inherited by a daughter cell after abscission are denoted by arrows and arrowheads, respectively. An example siVxn cell with incomplete cytokinesis eventually formed a bi-nucleated cell (bottom). The first image of a cell rounding up was considered time 0. For recorded cells with complete cytokinesis, the times required to reach (C) cleave furrow ingression and (D) cell abscission are expressed as mean ± SEM. (E) The percentage of recorded siCtrl and siVxn cells forming bi-nucleated cells. The cell numbers in each group used for analysis are indicated inside the bars. ** P < 0.01 by Student t test.
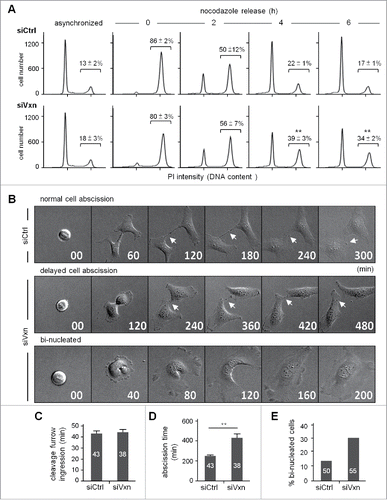
Figure 4. Expression of a SH3 domain-deleted mutant of vinexin increases the population of cells at midbody stage. (A) Illustration of various deletion mutants of vinexin β. (B) Thymidine-arrested cells were transfected with the plasmid expressing EGFP or EGFP-vinexin wild type (WT) or ΔSH3 mutants and then released for 14 h before α-tubulin immunostaining and Hoechst labeling. Quantification of green fluorescence intensity at the midbody stage from 20 cells expressing each EGFP construct. (C, D) Immunostaining of vinculin with (C) α-tubulin or (D) Vinexin at the midbody of HeLa cells. (E) HeLa cells infected with a lentivirus expressing control (siCtrl) or a shRNA against vinculin (siVCL) were harvested for immunoblotting. (F) Thymidine-arrested siCtrl and siVCL cells were released to fresh medium for 12 h, followed by immunostaining of vinexin and α-tubulin. The fluorescence intensity of vinexin at the midbody of HeLa cells was quantified from 20 cells per group. (G) Similar to (B), the images from 3 independent experiments were used to calculate the distribution of cells at the midbody stage and interphase. Arrows denote intercellular bridges. Scales, 10 μm. Data are mean ± SEM. ** P < 0.01, * P < 0.05 by Student t test.
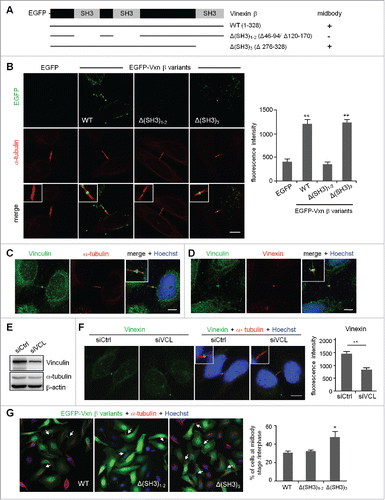
Figure 5. Midbody localization of rhotekin depends on vinexin. (A) Immunostaining of rhotekin (RTKN) and α-tubulin at the midbody of HeLa cells. (B) HeLa cells expressing myc-tagged rhotekin (myc-RTKN) were immunostained with vinexin and myc antibodies. Midbody dense structure observed by DIC microscopy. (C-E) siCtrl, siVxn and siRTKN-2 cells released from mitotic arrest were immunolabeled with the denoted antibodies. The fluorescence intensity of rhotekin, vinexin or RhoA-GTP at the midbody of HeLa cells was quantified from 20 cells per group. Data are mean ± SEM. ** P < 0.01 by Student t test.
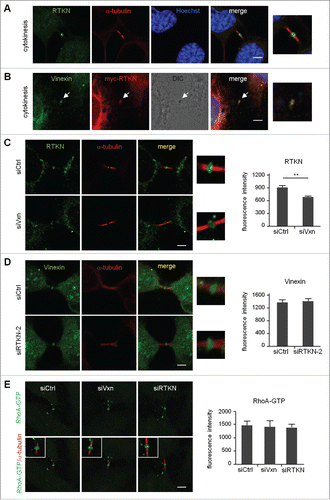
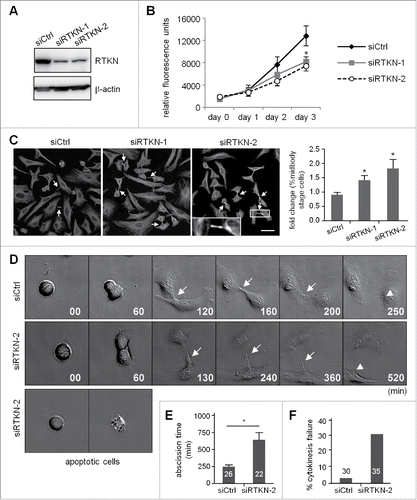
Figure 6. Knockdown of rhotekin impairs cell proliferation by cytokinectic delay and failure. (A) HeLa cells infected with a lentivirus expressing control (siCtrl) or shRNAs against human rhotekin (siRTKN-1, siRTKN-2) were harvested for immunoblotting of rhotekin and β-actin. (B) The proliferation rate of siCtrl and siRTKN cells monitored by Prestoblue assay. (C) Double thymidine-arrested siCtrl and siRTKN cells were released to fresh medium for 12 h, then underwent α-tubulin immunostaining and Hoechst labeling. The midbody stage is defined when 2 daughter cells are still connected by an intercellular bridge (arrows). Scale, 50 μm. The distribution of siCtrl or siVxn cells at the midbody stage and interphase is expressed as relative ratios with the ratio in siCtrl cells arbitrarily set to 1. Total cell number for each siRNA group from 3 independent experiments is ∼1000. (D) Time-lapse comparison of cytokinesis in a representative siCtrl cell (top) and siRTKN cell (middle). G1/S-synchronized siCtrl and siRTKN cells after release from the thymidine block were time-lapse recorded. Midbodies remained at the center of intercellular bridge and midbody remnants inherited by a daughter cell after abscission are denoted by arrows and arrowheads, respectively. Some siRTKN cells underwent apoptosis (bottom). The first image of a cell rounding up was considered time 0. (E) For recorded cells with complete cytokinesis, the time required to complete cell abscission is expressed as mean ± SEM. (F) The percentage of recorded siCtrl and siVxn cells with incomplete cytokinesis eventually forming a bi-nucleated cell. The cell numbers in each group used for analysis are labeled inside the bars. * P < 0.05 by Student t test.