Figures & data
Figure 1. Arsenic upregulates overall protein sumoylation in cells. (A) HeLa cells were treated with arsenic trioxide (ATO) at indicated concentrations for 24 h, after which cells were collected and lysed. Equal amounts of cell lysates were blotted with antibodies to SUMO2/3, cyclin B, UBC9 and ß-actin. Protein lysates from cells treated with nocodazole overnight were also used as control. (B) HeLa cells were treated with ATO (5 µM) for various times as indicated, after which cells were collected and lysed. Equal amounts of cell lysates were blotted with antibodies to SUMO2/3, UBC9 and ß-actin. (C) HCT116 cells were treated with ATO at indicated concentrations for 24 h, after which cells were collected and lysed. Equal amounts of cell lysates were blotted with antibodies to SUMO2/3, p53, and ß-actin. (D) HL-60 cells were treated with ATO at indicated concentrations for 24 h, after which cells were collected and lysed. Equal amounts of cell lysates were blotted with antibodies to SUMO2/3 and ß-actin.
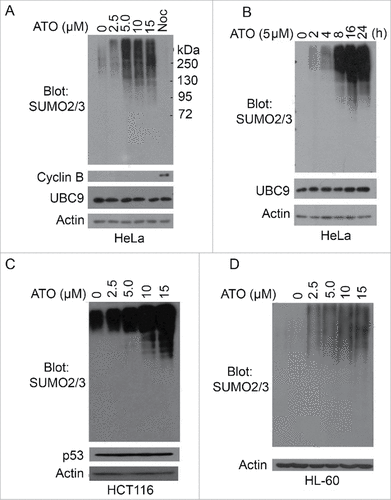
Table 1. Potential proteins with enhanced SUMO2-modification after As2O3 treatment.
Figure 2. Enhanced Mus81 sumoylation after arsenic treatment. (A) HeLa cells were treated with ATO (5 µM) for 24 h after which cells were collected for lysis and fractionations. Equal amounts of cytoplasmic and nuclear fractions, along with total cell lysates, were blotted with antibodies to Mus81, PARP1 and ß-actin. Mus81-S denotes SUMO-modified Mus81 bands. (B) HeLa cells were transfected with Mus81 siRNAs or luciferase siRNAs for 24 h after which equal amounts of cell lysates were blotted for Mus81 and ß-actin. (C) HeLa cells were co-transfected with plasmids expressing Flag-SUMO2, Flag-UBC9 and/or His6-Mus81 for 48 h after which cells were lysed. Equal amounts of lysates were incubated with Ni-IDA resin. Proteins bound to the resin, along with lysate inputs, were blotted with antibodies to Mus81, Flag, and ß-actin. (D) HeLa S2 cells that constitutively expressed transfected SUMO2 were transfected with a plasmid expressing GPF-Mus81 or GFP alone for 48 h after which equal amounts of lysates were immunoprecipitated with the anti-GFP antibody. GFP precipitates, along with lysate inputs, were blotted with antibodies to GFP, Mus81, and/or ß-actin. (E) HeLa cells were transfected with various plasmids as indicated for 48 h after which equal amoutns of lysates were precipitated with the anti-Mus81 antibody. Mus81 precipitates, along with lysate inputs, were blotted with antibodies to GFP, Mus81, and/or ß-actin.
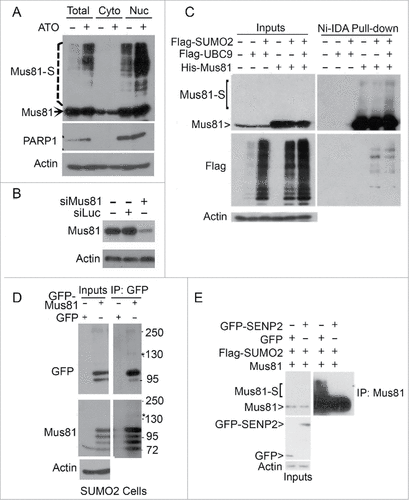
Figure 3. PIAS1 and PIAS3 promote Mus81 sumoylation. (A) HEK293 cells co-transfected with plasmids expressing Flag-SUMO2, His6-Mus81, and PIAS1 (or other family member) for 48 after which equal amounts of cell lysates were blotted with antibodies to Mus81, Flag, and ß-actin. (B) HEK293 cells were transfected with plasmids expressing various plasmid constructs as indicated for 48 h after which cells were lysed. Equal amounts of cell lysates were incubated with Ni-IDA resin after which proteins bound to the resin were eluted and blotted with the anti-Mus81 antibody. (C) HEK293 cells were co-transfected with plasmids expressing Flag-SUMO2, His6-Mus81, and Flag-PIAS3 (2 different concentrations) for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins bound to the resin, along lysate inputs, were blotted with antibodies to Mus81, Flag, and/or ß-actin.
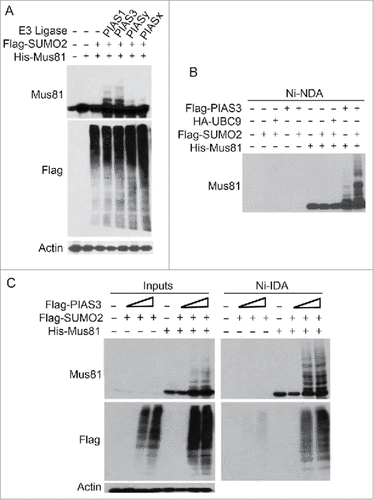
Figure 4. Lysines 10 and 524 are essential for Mus81 sumoylation. (A) Schematic presentation of Mus81 with 5 lysine residues that largely conform to sumoylation consensus motif. (B) HEK293 cells were transfected with various expression plasmid constructs as indicated for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins specifically bound to the resin were eluted and blotted, along with lysate inputs, for Mus81, Flag (SUMO2), Myc (PIAS3), and/or ß-actin. (C) HEK293 cells were transfected with various expression plasmid constructs as indicated for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins specifically bound to the resin were eluted and blotted, along with lysate inputs, for Mus81, Flag, and/or ß-actin.
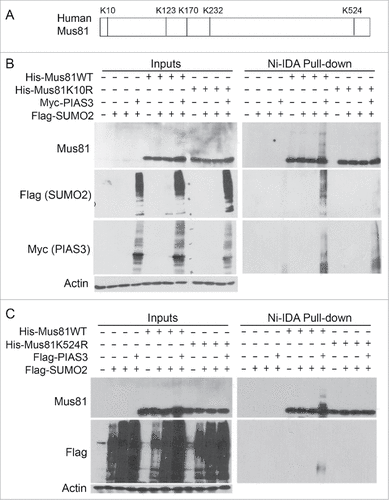
Figure 5. Expression of sumoylation-resistant Mus81 mutant induces lagging chromosomes and delays mitotic progression. (A) HEK293 cells were co-transfected with various expression plasmids as indicated for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins bound to the resin were eluted and blotted, along with lysate inputs, for Mus81, Flag, and/or ß-actin. (B) HeLa cells transfected with GFP-Mus81 or GFP-Mus81–5R for 48 h. Cells were then fixed and stained with DAPI. Mitotic cells were analyzed for the presence of mis-aligned/lagging chromosomes. (C) Mitotic cells with mis-aligned or lagging chromosomes (denoted with red star) after expression of either Mus81 or its sumoylation mutant as shown in B were counted. Data were summarized from 3 independent experiments. (D) HeLa cells transfected with His6-Mus81 or His6-Mus81–5R for 24 h after which cells were treated with nocodazole for 16 h. Mitotic cells were collected by shake-off and re-cultured in fresh medium for various times as indicated. Equal amounts of cell lysates were blotted for Mus81, cyclin B, and ß-actin.
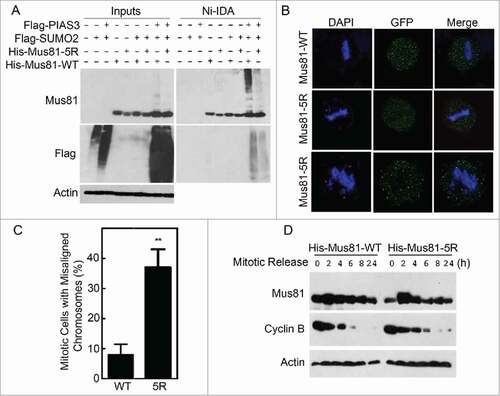
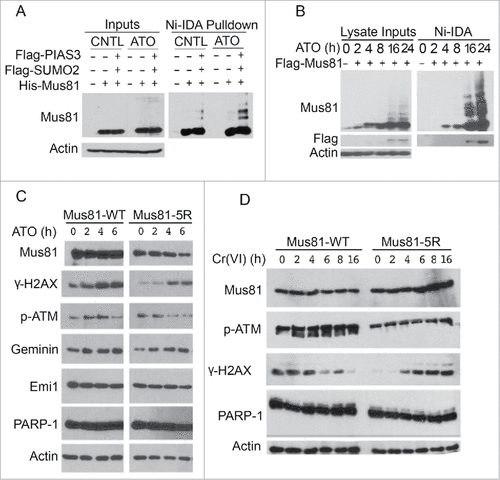
Figure 6. Cells expressing sumoylation-resistant mutant of Mus81 have compromised DNA damage responses to metal toxins. (A) HeLa cells were co-transfected with plasmids expressing for Flag-PIAS3, Flag-SUMO2, His6-Mus81 for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins specifically bound to the resin, along with lysate inputs, were blotted for Mus81 and/or ß-actin. (B) HeLa S2 cells were transfected with Mus81 expression construct for 24 h after which cells were treated with ATO for various times as indicated. Equal amounts of lysates were incubated with Ni-IDA resin. Protein specifically bound to the resin were eluted and blotted, along with lysate inputs, with antibodies to Mus81, Flag, and ß-actin. (C) HEK293 cells transfected with a plasmid construct expressing either Mus81 or Mus81–5R for 48 h after which cells were treated with ATO for various times. Equal amounts of cell lysates were blotted with antibodies to Mus81, γ-H2AX, p-ATM, Geminin, Emi1, PARP-1, and ß-actin. (D) HEK293 cells transfected with a plasmid construct expressing either Mus81 or Mus81–5R for 48 h after which cells were treated with Cr(VI) for various times as indicated. Equal amounts of cell lysates were then blotted with antibodies to Mus81, γ-H2AX, p-ATM, PARP-1, and ß-actin.