Figures & data
Figure 1. Distribution of fluorescence imaging. (A) S. typhimurium-A1-R-GFP targeting the osteosarcoma PDOX after intra-arterial (i.a.) injection. (B) S. typhimurium A1-R-GFP targeting the osteosarcoma after intravenous (i.v.) injection. Confocal microscopy imaging with the Olympus FV1000 demonstrated S. typhimurium A1-R-GFP targeting the osteosarcoma PDOX. Scale bars: 12.5 µm.
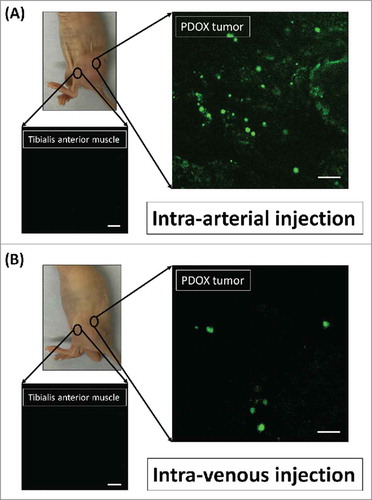
Figure 2. (A) Fluorescence intensity of S. typhimurium A1-R-GFP targeting the osteosarcoma PDOX after i.a. or i.v. administration. (B) Fluorescent area of S. typhimurium A1-R-GFP targeting the osteosarcoma PDOX after i.a. or i.v. administration.
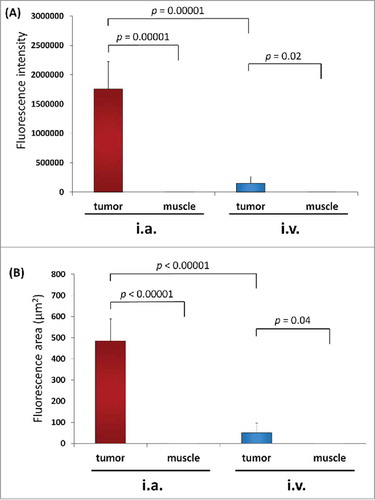
Figure 3. (A) Treatment schema. Mice were treated with CDDP, S. typhimurium A1-R i.v. or S. typhimurium A1-R i.a. CDDP (6 mg/kg/week i.p. for 2 weeks); S. typhimurium A1-R i.v. (5 × 107 CFU/100 μl, i.v., weekly, for 2 weeks); S. typhimurium A1-R i.a. (5 × 105 CFU/100 μl, i.a., once). Tumor volume was measured at the indicated time points after the onset of treatment. n = 8mice/group. (B) Treatment efficacy. *p < 0.05, **p < 0.001.
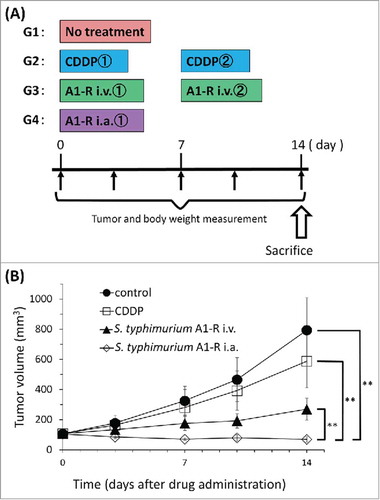
Figure 4. Body weight after various treatments. Bar graphs show body weight in each group at pre-treatment and 2 weeks after drug administration.
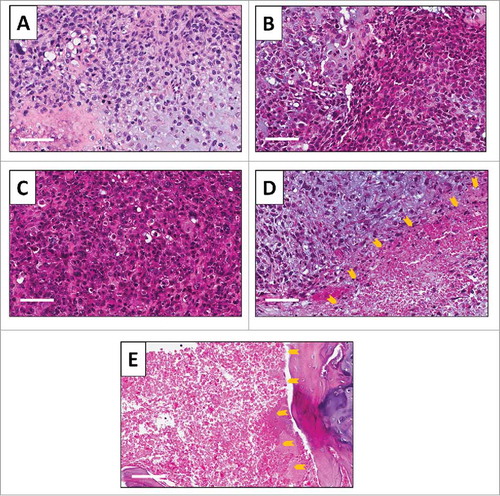
Figure 5. Tumor histology. Hematoxylin and eosin (H&E)-stained section of the (A) original patient tumor; (B) untreated PDOX tumor; (C) PDOX tumor treated with CDDP; (D) PDOX tumor treated with S. typhimurium A1-R i.v.; and (E) PDOX tumor treated with S. typhimurium A1-R i.a.. Necrotic areas are indicated by yellow arrows. White scale bars: 80 µm.