Figures & data
Figure 1. Increased ROS levels in oocyte from diabetes mice. GV and MII oocytes collected from control and diabetic mice were stained by CM-H2DCFAD (green) to evaluate ROS levels. (A and C) Representative images of CM-H2DCFAD fluorescence in oocytes from control and diabetic mice. Scale bar: 25 μm. (B and D) Quantitative analysis of fluorescence intensity shown in A and C (n = 9 oocytes for each group). Data were expressed as mean ± SD from 3 independent experiments. *p < 0.05 vs control.
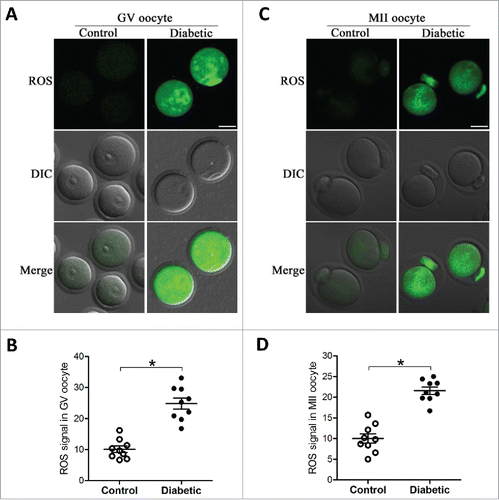
Figure 2. Reduced Sirt3 expression in oocyte from diabetes mice. Fully-grown GV oocytes were collected from control and diabetic mice, and then processed for immunoblotting. (A) Western blot analysis showed the reduced Sirt3 expression in oocytes from diabetic mice compared with controls. Tubulin served as an internal control. (B) Band intensity was measured by Image J software, and the ratio of Sirt3/Tubulin expression was normalized. All protein gel blot experiments were repeated at least 3 times, with a representative gel image shown.
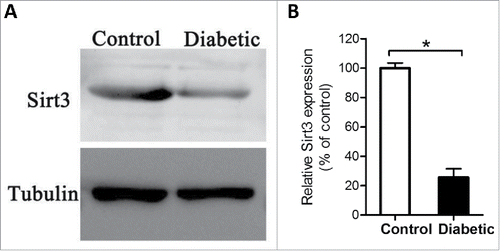
Figure 3. Sirt3 overexpression attenuates ROS production in oocyte. Exogenous Myc-Sirt3 mRNA or PBS was microinjected into fully-grown GV oocytes from diabetic mice, which were arrested at GV stage in M16 medium containing 2.5 μΜ milrinone for 20 hours to allow synthesis of new Myc-Sirt3 protein. Following in vitro-maturation, MII oocytes were stained by CM-H2DCFAD (green) to evaluate ROS levels. (A) Representative images of CM-H2DCFAD fluorescence in control, diabetic, diabetic+PBS and diabetic+Sirt3 oocytes. Scale bar: 25 μm. (B) Quantitative analysis of fluorescence intensity shown in B (n = 10 oocytes for each group). Data were expressed as mean ± SD from 3 independent experiments. *p < 0.05 vs control.
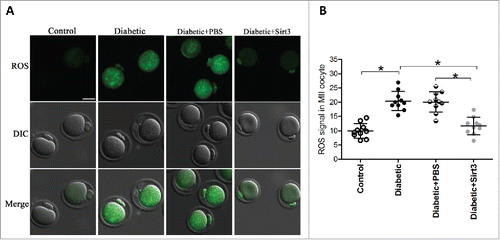
Figure 4. Sirt3 overexpression lowers the acetylation levels of SOD2K68 in oocytes from diabetic mice. Control (n = 77), diabetic (n = 71), diabetic+PBS (n = 80) and diabetic+Sirt3 (n = 76) MII oocytes were labeled with acetyl-SOD2K68 antibody and counterstained with Hoechst 33342 for chromosomes. (A) Representative images show the AcSOD2K68 signal and DNA in oocytes. Scale bar: 25 μm. (B) Quantitative analysis of fluorescence intensity shown in A. Data were expressed as mean ± SD from 3 independent experiments. *p < 0.05 vs control.
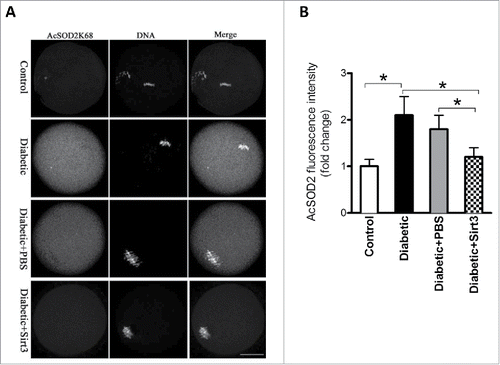
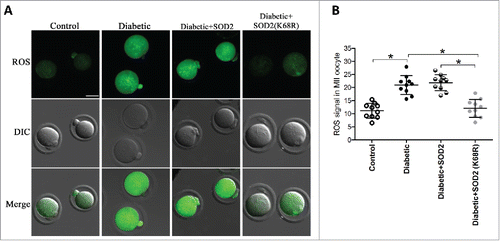
Figure 5. SOD2K68R partly prevents oxidative stress in oocytes from diabetic mice. SOD2 WT or SOD2K68R mutant mRNA was microinjected into fully-grown GV oocytes from diabetic mice, which were arrested at GV stage in M16 medium containing 2.5 μΜ milrinone for 20 hours to allow synthesis of new protein. Following in vitro-maturation, MII oocytes were stained with CM-H2DCFAD (green) to evaluate ROS levels. (A) Representative images of CM-H2DCFAD fluorescence in control, diabetic, diabetic+SOD2 and diabetic+SOD2 (K68R) oocytes. Scale bar: 25 μm. (B) Quantitative analysis of fluorescence intensity shown in A (n = 10 oocytes for each group). Data were expressed as mean ± SD from 3 independent experiments. *p < 0.05 vs control.