Figures & data
Figure 1. Post-transcriptional histone modifications in centrochromatin. In centromere regions, CENP-A nucleosomes are interspersed with chromatin-containing canonical histone H3 and this composite organization must be critical for the formation of centromere-specific chromatin called ‘centrochromatin’. Various histones are modified in centromeres and these modifications contribute to centromere functions including centromere specification, centromere transcription, heterochromatin formation, kinetochore assembly.
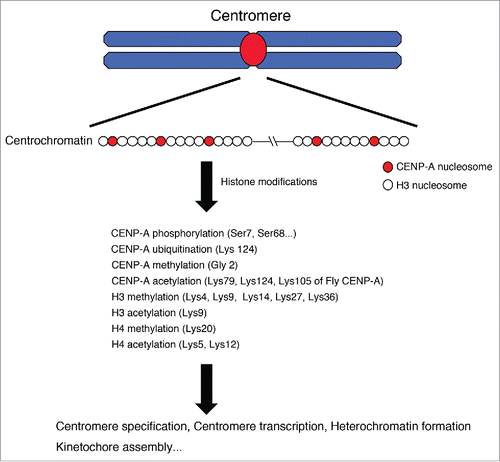
Figure 2. Schematic model for the functional role of H4K20me1 in centrochromatin. The CENP-A-H4 complex is associated with the CENP-A chaperone HJURP, and has been shown to be incorporated into centromeres during early G1 phase of the cell cycle. Given that H4 subunits in CENP-A nucleosomes have been found to be consistently methylated at K20, it is likely that this modification occurs just after CENP-A incorporation by PR-Set7. This would allow various Constitutive-Centromere-Associated-Network (CCAN) proteins to recognize H4K20me1 in centrochromatin, and thereby assemble kinetochores. It is vital that future research elucidates the timing and mechanisms by which CCAN proteins mediate kinetochore assembly.
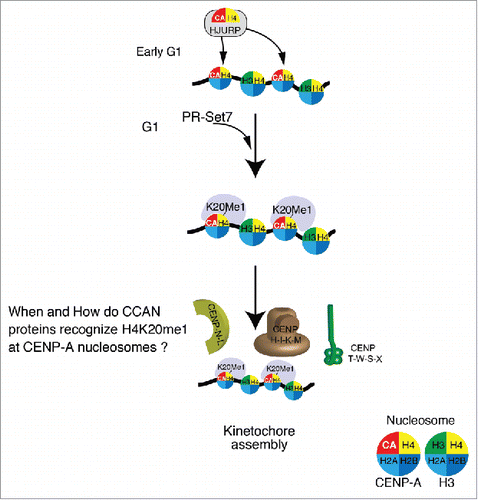
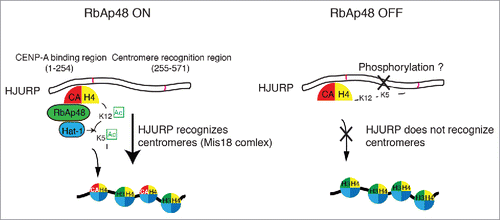
Figure 3. Role for the RbAp48-Hat1 complex-mediated acetylation of the H4 tail in CENP-Adeposition. The H4 tail in the CENP-A-H4 complex is acetylated at K5 and K12 by the RbAp48-Hat1 complex before H4-CENP-A centromere deposition. The CENP-A-H4 complex binds the N-terminus of HJURP, and the middle region of HJURP recognizes centromeres (via the Mis18 complex) to facilitate centromere deposition. Acetylation of the H4 tail normally facilitates this process; however, if acetylation does not occur correctly (as in RbAp48-deficient cells), the non-acetylated tail interferes with HJURP centromere recognition, preventing CENP-A from being incorporated into centromere domains. It is unclear how H4 acetylation facilitates CENP-A deposition. Given that HJURP is a highly phosphorylated protein, it may be that a combination of HJURP phosphorylation and H4 acetylation is critical for correct H4-CENP-A deposition.