Figures & data
Figure 1. CP110 is phosphorylated by PLK4 in vitro. (A) Summary of the in vitro kinase assays of PLK4. The red lines indicate the full-length or truncated CP110 proteins that were phosphorylated in vitro by PLK4. (B) In vitro kinase assays were performed with truncated fragments of GST-CP110 spanning the 1–184 residues. The wild type (WT) or kinase dead (KD) forms of GST-PLK4-kido (kinase domain) were used as enzymes. (C) In vitro kinase assays were performed with the GST-CP11041–109 proteins with alanine substitution mutations at the presumptive phosphorylation sites. (D) The full-length GST-CP110 (WT) and GST-CP110S98A (S98A) fusion proteins were finally tested for in vitro phosphorylation by GST-PLK4-kido. Protein levels were determined by Coomassie blue staining.
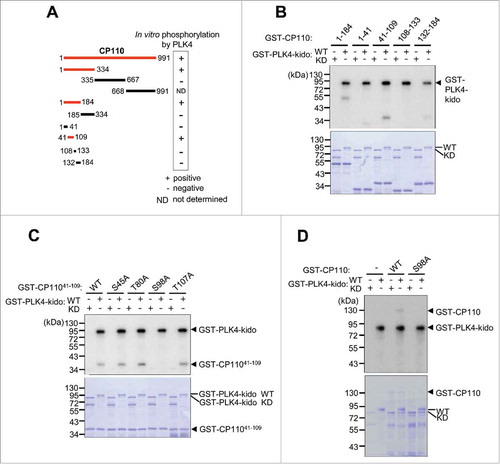
Figure 2. CP110 is phosphorylated by PLK4 in vivo. (A) CP110-depleted U2OS cells were treated with thymidine for 20 h and coimmunostained with the centrin-2 antibody (green) and CP110 or pS98CP110 antibodies (red). The centrosomal intensities of CP110 and pS98CP110 were determined in greater than 30 centrosomes per experimental groups in 3 independent experiments. (B) The PLK4-depleted HeLa cells were arrested at M phase with the STLC treatment of 3 h and coimmunostained with antibodies specific to centrin-2 (green) and pS98CP110 (red). The centrosomal intensities of pS98CP110 were determined in greater than 30 centrosomes per experimental groups in 3 independent experiments. (C) The PLK4-depleted HeLa cells were lysed and subjected to immunoprecipitation with anti-CP110 serum. Immunoblot analysis was performed with the CP110 and pS98CP110 antibodies. Preimmune serum (PI) was used as a control. A larger version of the blot images is presented in Fig. S3. (D) The M phase-arrested HeLa cells were synchronously released to G1 phase, cultured for up to 12 h and coimmunostained with γ-tubulin antibody (red) along with the CP110 or pS98CP110 antibodies (green). Greater than 10 centrosomes per experimental groups were assessed. (E) HeLa cells were coimmunostained with γ-tubulin antibody (red) along with CP110 or pS98CP110 antibodies (green). Mitotic stages were determined with DAPI (blue). Greater than 10 centrosomes per experimental groups were determined. Values are means and standard deviations. The statistical significance was determined by paired t-tests. *, P<0.05.
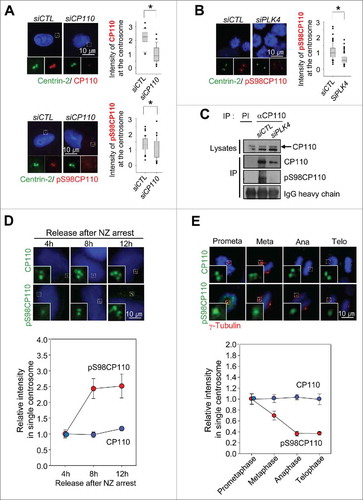
Figure 3. Inhibitory effects of the phospho-resistant Flag-CP110S98A mutant on centriole duplication. (A) U2OS cells were transiently transfected with pFlag-GFP, pFlag-CP110WT, pFlag-CP110S98A or pFlag-CP110S98E. The transfected cells were treated with thymidine for 20 h. Expression of ectopic CP110 proteins was confirmed by immunoblot analysis. The asterisk indicates a nonspecific band. The cells were coimmunostained with antibodies specific to Flag (green) and centrin-2 (red). DNA was stained with DAPI (blue). The number of centrioles per cell was counted based on centrin-2 signals. Greater than 200 cells per experimental group were analyzed in 4 independent experiments. (B) Endogenous CP110 was depleted in U2OS cells. After siRNA transfection, the cells were rescued with siRNA-resistant constructs of pFlag-CP110WT, pFlag-CP110S98A or pFlag-CP110S98E. The cells were then treated with hydroxyurea (HU) for 72 h. Expression of endogenous CP110 and ectopic CP110 proteins was confirmed by immunoblot analysis. The cells were coimmunostained with antibodies specific to Flag (green) and γ-tubulin (red). DNA was stained with DAPI (blue). The centrosome number was assessed based on γ-tubulin signals. Greater than 300 cells per experimental group were analyzed in 3 independent experiments. Values are means and standard deviations. *, P <0.05.
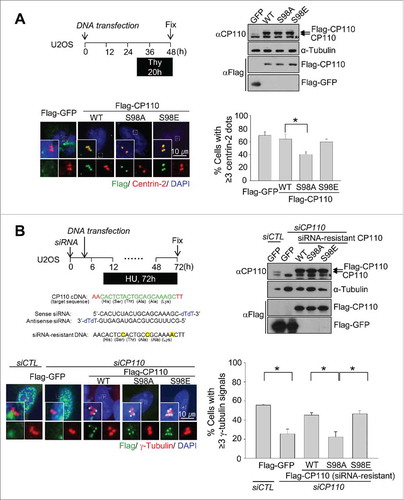
Figure 4. Induction of centriole duplication with the phospho-mimetic CP110S98E mutant in PLK4-reduced conditions. (A) Expression of ectopic Flag-GFP, Flag-GFP-CP110WT, Flag-GFP-CP110S98A or Flag-GFP-CP110S98E was induced in Tet-on U2OS stable cell lines. Endogenous PLK4 was depleted with siRNA transfections. Immunoblot analysis confirmed the expression of ectopic CP110 proteins. The cells were treated with hydroxyurea for 26 h and coimmunostained with antibodies specific to Flag (green) and centrin-2 (red). DNA was stained with DAPI (blue). The number of centrioles per cell was counted. Greater than 200 cells per experimental group were analyzed in 3 independent experiments. (B) Flag-GFP-, Flag-GFP-CP110WT- and Flag-GFP-CP110S98E-expressing U2OS cells were treated with 100 nM centrinone, a PLK4 inhibitor, for 36 h and coimmunostained with antibodies specific to Flag (green) and centrin-2 (red). DNA was stained with DAPI (blue). Greater than 180 cells per experimental group were analyzed in 3 independent experiments. Values are means and standard deviations. *, P<0.05.
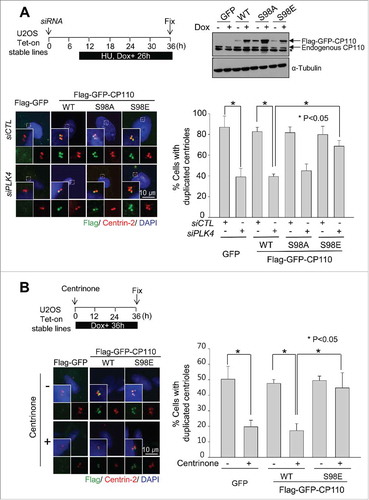
Figure 5. Augmentation of the centrosomal SAS6 with the phospho-mimetic CP110S98E mutant. (A) Flag-GFP-, Flag-GFP-CP110-, Flag-GFP-CP110S98A- and Flag-GFP-CP110S98E-expressing U2OS cells were treated with 100 nM centrinone for 48 h and coimmunostained with antibodies specific to SAS6 (red) and Flag (green). DNA was stained with DAPI (blue). Relative intensities of the centrosomal SAS6 were measured from 100 centrosomes in 2 independent experiments and analyzed with box-and whisker plots. The bottom and top of each box mark the 25th and 75th percentiles, respectively. The whiskers are drawn down to the 5th percentile and up to the 95th. Points that lie outside the 5th and 95th percentiles are plotted as symbols. *P < 0.05, paired t-test. (B) U2OS stable cell lines were transfected with siSAS6. Forty-eight hours later, the cells were subjected to immunoblot analysis to confirm depletion of SAS6. The cells were also immunostained with the centrin-2 antibody to determine the number of centrioles. Greater than 100 cells were counted per experimental group in 3 independent experiments. Values are means and standard deviations.
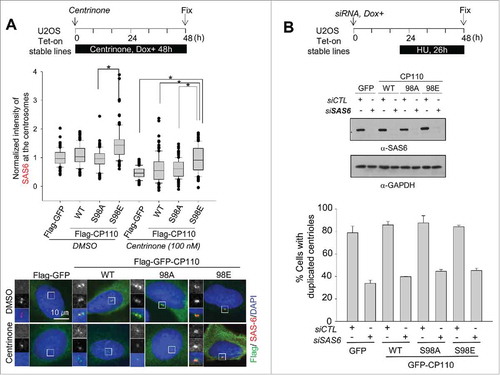
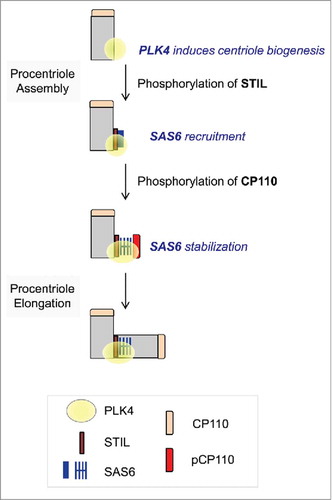
Figure 6. Model. Centriole assembly initiates at the proximal end of mother centriole where PLK4 is concentrated. STIL phosphorylation by PLK4 mediates SAS6 recruitment.Citation28,Citation29 CP110 is also phosphorylated by PLK4. This event may stabilize SAS6 during the initial procentriole assembly.