Figures & data
Figure 1. S109 phosphorylation of ARPP19 neither prevents its S67 phosphorylation by Gwl nor its ability to activate Cdk1. A. WT-ARPP was incubated with PKA, Gwl or both kinases in the presence of γS-ATP. The phosphorylation of WT-ARPP at S67 and at S109 was monitored by western blot using antibodies directed against S109-phosphorylated ARPP (pS109-ARPP) and S67-phosphorylated ARPP (pS67-ARPP). Total ARPP19 was immunoblotted with an anti-GST antibody (GST-ARPP). B. Prophase-arrested oocytes were stimulated with progesterone (Pg) or injected with in vitro thiophosphorylated ARPP19 at either S109 (pS109-ARPP), at S67 (pS67-ARPP) or both sites (pS67-pS109-ARPP). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. C. Prophase-arrested oocytes were injected or not with p21Cip1 (Cip1), and then with in vitro thiophosphorylated ARPP19 at S109 (pS109), at S67 (pS67) or at both sites (pS67-pS109). Oocytes were collected at the time of GVBD and ARPP19 proteins were GST-pulled down. Cdk1 activation was monitored in supernatants by immunoblotting phosphorylated Cdk substrates (pCdk substrates).
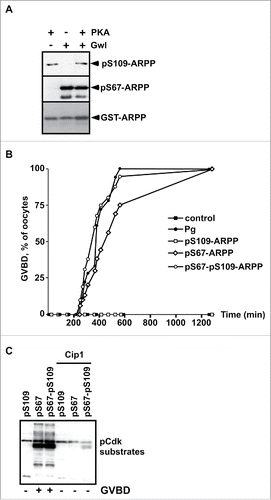
Figure 2. S109D mutation does not impair the ability of S67-phosphorylation of ARPP19 to activate Cdk1. A. WT-ARPP, S109A-ARPP and S109D-ARPP were incubated or not with recombinant Gwl in the presence of γS-ATP. The phosphorylation of WT-ARPP at S67 was visualized by western blot using an antibody directed against S67-phosphorylated ARPP (pS67-ARPP). Total ARPP19 was immunoblotted with an anti-GST antibody (GST-ARPP). B. Prophase-arrested oocytes were stimulated with progesterone (Pg) or injected with either unphosphorylated WT-ARPP (ARPP), S109A-ARPP and S109D-ARPP or S67-thiophosphorylated WT-ARPP, S109A-ARPP and S109D-ARPP (respectively pS67-ARPP, pS67-S109A-ARPP and pS67-S109D-ARPP). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. C. Prophase-arrested oocytes were injected or not with p21Cip1 (Cip1) and then stimulated with progesterone (Pg) or by injecting S67-phosphorylated WT-ARPP, S109A-ARPP or S109D-ARPP (respectively pS67, pS67-S109A, pS67-S109D). Oocytes were collected at GVBD time. Cdk1 activation was monitored by western blotting phosphorylated MAPK (pMAPK) and Cdc27.
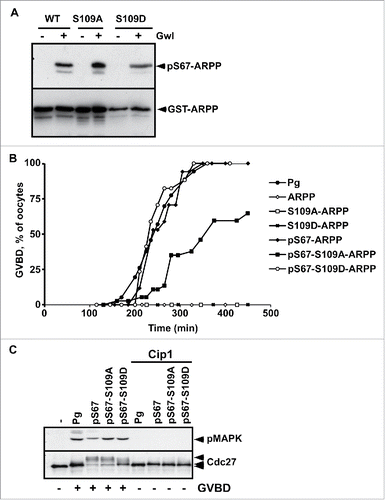
Figure 3. S67-phosphorylated ARPP19 interacts with PP2A-B55δ independently of its S109 phosphorylation. A. Prophase-arrested oocytes were injected or not with p21Cip1 (Cip1) and then injected with thiophosphorylated ARPP at either S109 (pS109), or S67 (pS67) or both residues (pS67-pS109). Oocytes were collected at the time of GVBD and ARPP19 proteins were GST-pulled down. GST-pulled down fractions were immunoblotted for PP2A-C ανδ B55δ subunits. Total ARPP19 was immunoblotted using a GST antibody (GST-ARPP). B. Prophase-arrested oocytes were induced to mature with progesterone (Pg) or by injecting S67-phosphorylated WT-, S109A- or S109D-ARPP (respectively pS67, pS67-S109A, pS67-S109D). Oocytes were collected at the time of GVBD and ARPP19 proteins were GST-pulled down. GST-pulled down fractions were immunoblotted for B55δ, PP2A-C subunits and total ARPP19 using a GST antibody (GST-ARPP).
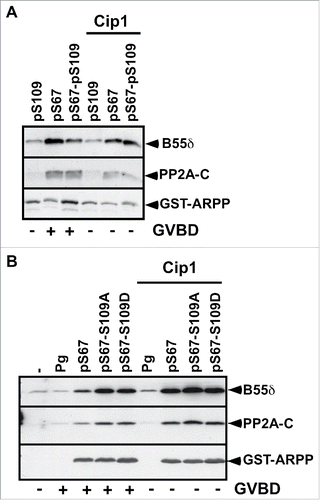
Figure 4. S28D mutation of ARPP19 does not induce meiosis resumption. A. Prophase-arrested oocytes were injected with 100 ng of either WT-ARPP19 (ARPP), S28A-ARPP or S28D-ARPP and then stimulated or not with progesterone (Pg). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. B. Oocytes from panel (A) were collected at the time of GVBD and Cdk1 activation was analyzed by western blotting Cyclin B2, Y15-phosphorylated Cdk1 (pY15-Cdk1) and total ARPP19 (GST-ARPP).
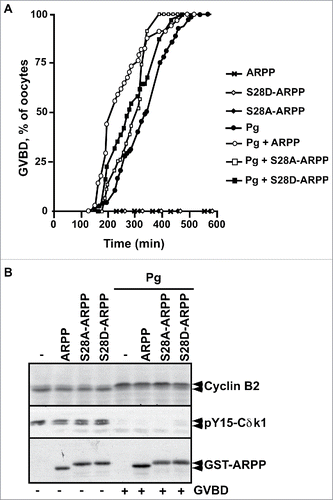
Figure 5. The phosphorylation of ARPP19 by Cdk1 is not essential for meiosis resumption. A. Prophase-arrested oocytes were injected or not with either unphosphorylated WT-ARPP19 (ARPP) or S28A-ARPP or Cdk1-thiophosphorylated forms of ARPP: WT-ARPP (pCdk1-ARPP) or S28A-ARPP (pCdk1-S28A-ARPP). Oocytes were then stimulated or not with progesterone (Pg). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. B. Oocytes from panel (A) were collected at the time of GVBD and Cdk1 activation was analyzed by western blotting Cyclin B2, S287-phosphorylated Cdc25 (pS287-Cdc25), phosphorylated MAPK (pMAPK) and total ARPP19 (GST-ARPP). C. Prophase-arrested oocytes were injected with single S67-phosphorylated ARPP (pS67-ARPP), or single Cdk1-thiophosphorylated forms of either WT-ARPP19 (pCdk1-ARPP) or S28A-ARPP (pCdk1-S28A-ARPP) or with double Cdk1 and Gwl phosphorylated ARPP proteins: WT-ARPP19 (pS67-pCdk1-ARPP) or S28A-ARPP (pS67-pCdk1-S28A-ARPP). As controls, oocytes were stimulated with progesterone (Pg). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. D. Oocytes from panel (C) were collected at the time of GVBD and Cdk1 activation was analyzed by western blotting Cyclin B2, S287-phosphorylated Cdc25 (pS287-Cdc25), phosphorylated MAPK (pMAPK), Y15-phosphorylated Cdk1 (pY15-Cdk1) and total ARPP19 (GST-ARPP).
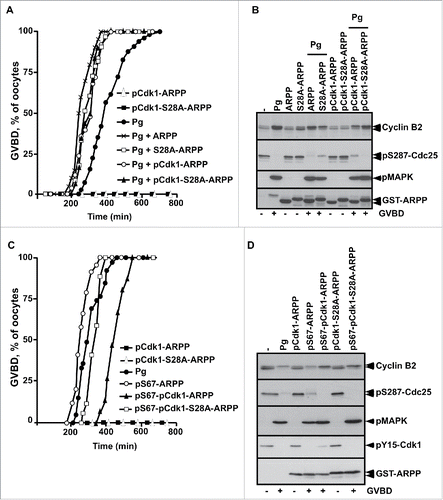
Figure 6. ARPP19 phosphorylation at S109 does not impair meiosis resumption triggered by S67-phosphorylated ARPP and the subsequent inhibition of PP2A-B55δ. A. Prophase-arrested oocytes were injected or not with 150 ng or 300 ng of S67A-S109D-ARPP and then stimulated either with progesterone (Pg) or by injecting S67-thiophosphorylated S109A-ARPP (pS67-S109A-ARPP, 150 ng per oocyte). Meiosis resumption was followed by scoring the % of GVBD 18 hours after hormonal stimulation or pS67-S109A-ARPP injection. B. Cdk1 activation was analyzed in oocytes from panel (A) by western blotting Gwl, S287-phosphorylated Cdc25 (pS287-Cdc25), phosphorylated MAPK (pMAPK) and Y15-phosphorylated Cdk1 (pY15-Cdk1).
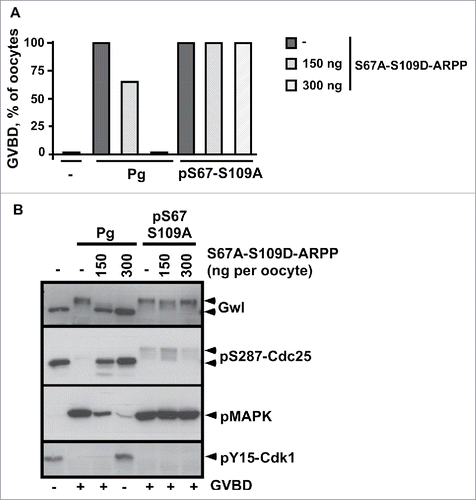
Figure 7. Schematic representation of the regulation of meiosis resumption by ARPP19 in Xenopus oocytes. ARPP19 is phosphorylated by PKA at S109 in prophase-arrested oocytes. The molecular targets of the single S109-phosphorylated form of ARPP19 that are responsible for the prophase arrest are unknown. Our study shows that S109 phosphorylation does not prevent ARPP19 phosphorylation at S67 by Gwl. In response to progesterone, PKA activity drops down and consequently, ARPP19 is dephosphorylated at S109. This event unlocks a signaling pathway that generates a threshold activity of Cdk1. Once this starter amount of Cdk1 is formed, it induces Gwl activation that in turn phosphorylates ARPP19 at S67. As a consequence, PP2A-B55δ is inhibited and the MPF auto-amplification loop is launched. Moreover, ARPP19 is also re-phosphorylated at S109 by an unknown kinase distinct of PKA. This phosphorylation contributes to M-phase entry. Hence, the active form of ARPP19 that sustains Cdk1 activation does not only rely on phosphorylation at S67 as previously thought, but also on its concomitant phosphorylation at S109.