Figures & data
Figure 1. (A) eIF4A1 is the major Pdcd4 binding partner in NCI-H1299 cells. NCI-H1299[E-Pdcd4] clonal derivative of NCI-H1299 cells (ATCC #CRL-5803) was obtained by transfection of the cells with pEGFP-Pdcd4 plasmidCitation21 with subsequent selection of clones stably producing EGFP-Pdcd4 protein. Lysates of NCI-H1299[E-Pdcd4] (lane 1) or parental NCI-H1299 cells (lane 2) were prepared in the buffer (25 mM Tris-HCl, pH 7.4; 250 mM NaCl; 5 mM EDTA; 1% NP-40; 1x Protease inhibitor cocktail (Sigma, St. Louis, MO, USA)) and incubated with anti-EGFP monoclonal antibody-coated beads (Proteinsynthesis, Moscow, Russia). After washing with phosphate buffered saline, captured proteins were eluted in SDS-PAAG loading buffer by boiling and separated in 10% SDS-PAAG. The gel was stained with Bio-Safe Coomassie stain (Bio-Rad, Hercules, CA, USA). Approximately 50 kDa protein band (marked by an arrow) co-purified with EGFP-Pdcd4 protein (marked by an asterisk) from NCI-H1299[E-Pdcd4] cell extract was excised from the gel and identified as human eIF4A1 protein based on results of MALDI-TOF mass-spectrometry. MW – protein molecular weight marker, with protein molecular weights indicated in kDa on the right. (B) Evaluation of relative Pdcd4 and eIF4A1 protein abundances in cancerous NCI-H1299 cells. Lysate of NCI-H1299[E-Pdcd4] cells was incubated with anti-EGFP monoclonal antibody-coated beads. After incubation, bead washing and protein elution from beads as described in (A), proportional amounts of initial lysates (lanes 1), eluted proteins (lanes 2) and flow-through (lanes 3) were analyzed by Western blotting with anti-Pdcd422 or anti-eIF4A1 (Cell Signaling, Boston, MA, USA) rabbit antibodies. EGFP-Pdcd4 and endogenous Pdcd4 protein bands are marked by an asterisk and by an arrow, respectively. (C) Evaluation of relative Pdcd4 and eIF4A1 protein abundances in non-cancerous HBEpC cells. Lysate of HBEpC cells (EACCC #502-05a) infected with recombinant replication-deficient adenovirus for EGFP-Pdcd4 protein production (constructed using Ad-Easy system (Stratagene, La Jolla, CA, USA) with derived from pEGFP-Pdcd4 plasmid expression module consisting from CMV promoter-driven EGFP-Pdcd4 cDNA with SV40 virus transcription termination and poyadenylation signal) was incubated with anti-EGFP monoclonal antibody-coated beads. After incubation, bead washing and protein elution from beads as described in (A), proportional amounts of initial lysates (lanes 1), eluted proteins (lanes 2) and flow-through (lanes 3) were analyzed by Western blotting with anti-Pdcd421 or anti-eIF4A1 (Cell Signaling, Boston, MA, USA; Cat. #2490) rabbit antibodies. EGFP-Pdcd4 protein band is marked by an asterisk.
![Figure 1. (A) eIF4A1 is the major Pdcd4 binding partner in NCI-H1299 cells. NCI-H1299[E-Pdcd4] clonal derivative of NCI-H1299 cells (ATCC #CRL-5803) was obtained by transfection of the cells with pEGFP-Pdcd4 plasmidCitation21 with subsequent selection of clones stably producing EGFP-Pdcd4 protein. Lysates of NCI-H1299[E-Pdcd4] (lane 1) or parental NCI-H1299 cells (lane 2) were prepared in the buffer (25 mM Tris-HCl, pH 7.4; 250 mM NaCl; 5 mM EDTA; 1% NP-40; 1x Protease inhibitor cocktail (Sigma, St. Louis, MO, USA)) and incubated with anti-EGFP monoclonal antibody-coated beads (Proteinsynthesis, Moscow, Russia). After washing with phosphate buffered saline, captured proteins were eluted in SDS-PAAG loading buffer by boiling and separated in 10% SDS-PAAG. The gel was stained with Bio-Safe Coomassie stain (Bio-Rad, Hercules, CA, USA). Approximately 50 kDa protein band (marked by an arrow) co-purified with EGFP-Pdcd4 protein (marked by an asterisk) from NCI-H1299[E-Pdcd4] cell extract was excised from the gel and identified as human eIF4A1 protein based on results of MALDI-TOF mass-spectrometry. MW – protein molecular weight marker, with protein molecular weights indicated in kDa on the right. (B) Evaluation of relative Pdcd4 and eIF4A1 protein abundances in cancerous NCI-H1299 cells. Lysate of NCI-H1299[E-Pdcd4] cells was incubated with anti-EGFP monoclonal antibody-coated beads. After incubation, bead washing and protein elution from beads as described in (A), proportional amounts of initial lysates (lanes 1), eluted proteins (lanes 2) and flow-through (lanes 3) were analyzed by Western blotting with anti-Pdcd422 or anti-eIF4A1 (Cell Signaling, Boston, MA, USA) rabbit antibodies. EGFP-Pdcd4 and endogenous Pdcd4 protein bands are marked by an asterisk and by an arrow, respectively. (C) Evaluation of relative Pdcd4 and eIF4A1 protein abundances in non-cancerous HBEpC cells. Lysate of HBEpC cells (EACCC #502-05a) infected with recombinant replication-deficient adenovirus for EGFP-Pdcd4 protein production (constructed using Ad-Easy system (Stratagene, La Jolla, CA, USA) with derived from pEGFP-Pdcd4 plasmid expression module consisting from CMV promoter-driven EGFP-Pdcd4 cDNA with SV40 virus transcription termination and poyadenylation signal) was incubated with anti-EGFP monoclonal antibody-coated beads. After incubation, bead washing and protein elution from beads as described in (A), proportional amounts of initial lysates (lanes 1), eluted proteins (lanes 2) and flow-through (lanes 3) were analyzed by Western blotting with anti-Pdcd421 or anti-eIF4A1 (Cell Signaling, Boston, MA, USA; Cat. #2490) rabbit antibodies. EGFP-Pdcd4 protein band is marked by an asterisk.](/cms/asset/7b602048-49fe-4ca2-bac3-1b15d8cfa3db/kccy_a_1371881_f0001_b.gif)
Figure 2. eIF4A is not substantially up-regulated in lung cancer cell lines compared to primary human bronchial epithelial cells HBEpC. eIF4A (eIF4A) and eIF4A1 (eIF4A1) proteins were detected by Western blotting with the respective antibodies (Cell Signaling; Cat. #2013 and Cat. #2490, respectively) in total cell lysates of indicated lung cancer cell lines or HBEpC cells. Upper panel shows level of Pdcd4 protein in the lysates of the indicated cell lines detected with anti-Pdcd421 antibodies (Pdcd4). The membrane was also probed with DM1α monoclonal anti-α-tubulin antibodies (Sigma, St. Louis, MO, USA; Cat. #T6199) (α-tubulin) to monitor total protein loading.
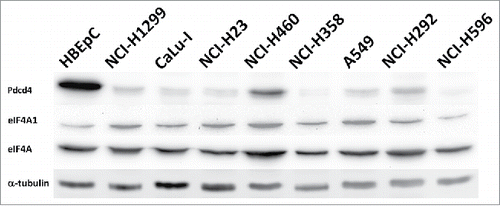
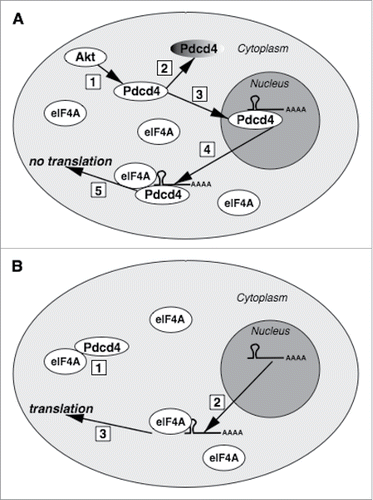
Figure 3. The proposed model of Pdcd4 action. (A) Upon Akt signaling activation (1), Pdcd4 will be targeted for proteasomal degradation (2) and translocated into the nucleus (3). In the nucleus, Pdcd4 can bind its mRNA targets. If translocated from the nucleus to the cytoplasm (4) which might be triggered by unknown stimuli, Pdcd4:mRNA complex would bind eIF4A but, despite abundant eIF4A in the cytoplasm, initial complex formation between Pdcd4 and its target mRNA in the nucleus would preclude from translation (5). (B) Due to excess of eIF4A over Pdcd4, cytoplasmic Pdcd4 would be sequestered by eIF4A (1), with the remaining abundant pool of eIF4A protein capable to support translation from Pdcd4 target mRNAs (3) exported from the nucleus (2).