Figures & data
Figure 1. NVP-BEZ235 inhibited PI3K/AKT/mTOR pathway and proliferation of neuroblastoma cells. (A, C) SH-SY5Y and SK-N-MC cells were treated with 0, 10, 25, 50, 100, 200, 500 and 1000 nM NVP-BEZ235 for 24 h. Cell viability was determined by CCK-8 assay. (B, D) Western blot analysis for the expression of p-AKT, AKT, p-p70S6K, p70S6K, p-MTOR, p-4E-BP1 in SH-SY5Y and SK-N-MC cells treated with 500 and 1000 nM NVP-BEZ235 for 12 h and 24 h. (E, F) Morphological changes in SH-SY5Y and SK-N-MC cells treated with NVP-BEZ235 (500, 1000 nM) for 24 h. (G, H) Western blot analysis for the expression of M CM2 in SH-SY5Y and SK-N-MC cells treated with 500 and 1000 nM NVP-BEZ235 for 12 h and 24 h.
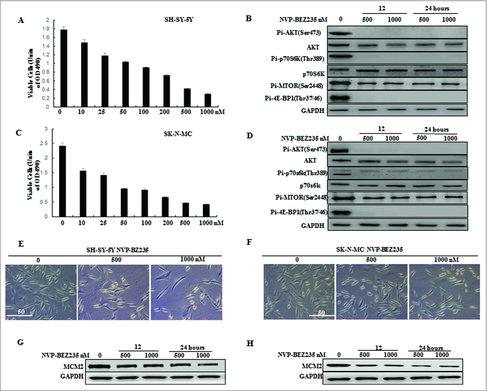
Figure 2. NVP-BEZ235 induced G0/G1 cell cycle arrest but not apoptosis in neuroblastoma cells. (A, B) Morphological changes in SH-SY5Y and SK-N-MC cells treated with NVP-BEZ235 (500, 1000 nM) for 24 h followed by AO/EB staining (magnification, 100 ×). Normal cells showed green fluorescence by acridine orange (AO) staining, apoptotic cells showed yellow-orange fluorescence by merge of ethidium bromide (EB) staining and AO staining. (C, D) Western blot analysis for cleaved caspase-3 and cleaved PARP in SH-SY5Y and SK-N-MC cells treated with serial NVP-BEZ235 for 24 h. (E, F) SH-SY5Y and SK-N-MC cells were treated with NVP-BEZ235 (100, 200 nM) for 24 h. Cell cycles were detected by flowcytometry analysis which determining cellular DNA content with hypotonic PI solution. (G, H) SH-SY5Y and SK-N-MC cells were treated with NVP-BEZ235 (200 nM) for the indicated times. Cyclin D1 and cyclin E1 proteins were performed by western blot analysis.
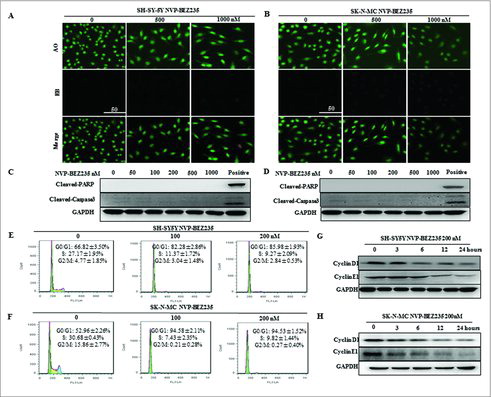
Figure 3. NVP-BEZ235 decreased cyclin D1 and cyclin E1 levels through enhancing their protein degradation. (A, B) SH-SY5Y and SK-N-MC cells were incubated with NVP-BEZ235 for the indicated time periods. Cyclin D1 and cyclin E1 mRNA levels were determined by Q-RT-PCR. (C-J) SH-SY5Y and SK-N-MC cells were pre-treated with vehicle or 200 nM NVP-BEZ235 for 1 h, then adding 10 μg/ml CHX for another various times and cyclin D1 and cyclin E1 protein expression levels were detected by immunoblotting and quantified by the densitometry analysis and normalized against β-Actin expression.
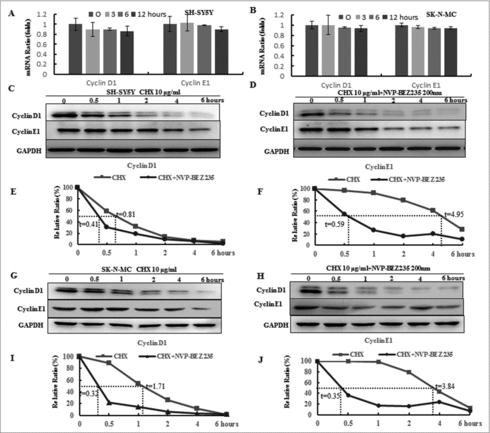
Figure 4. The ubiquitin proteasome pathway involved in NVP-BEZ235- induced the degradation of cyclin D1 and cyclin E1. (A-D) SH-SY5Y and SK-N-MC were pretreated for 1 h with autophagy inhibitor 20 μM CQ and subsequently treated for another 12 hours with 200 nM NVP-BEZ235. Western blot analyzed cyclin D1 and cyclin E1. (E-H) SH-SY5Y and SK-N-MC cells were pretreated for 1 h with 10 μM proteasome inhibitor (MG132) and subsequently treated for another 12 hours with 200 nM NVP-BEZ235 and lysates were probed for cyclin D1 and cyclin E1.
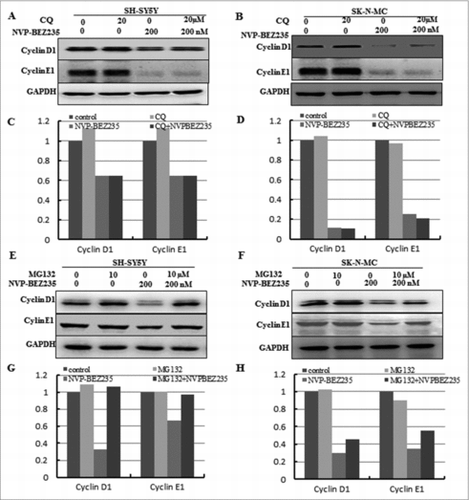
Figure 5. GSK3β is required for NVP-BEZ235- induced degradation of cyclin D1 and cyclin E1. (A and B) SH-SY5Y and SK-N-MC cells were incubated with NVP-BEZ235 for the indicated time periods. Western blot analyzed p-GSK3β and GSK3β. (C, D) SH-SY5Y and SK-N-MC were pretreated with GSK3β inhibitor LiCl for 1 h and then incubated with 200 nM NVP-BEZ235 for 12 h. Western blot analyzed cyclin D1 and cyclin E1. (E and F) SH-SY5Y and SK-N-MC were transfected with the siRNA targeting GSK3β for 48 h and then treated with 200 nM NVP-BEZ235 for another 12 h. The cells were lysed and analyzed by immunoblotting against cyclin D1 and cyclin E1. (G and H) SH-SY5Y and SK-N-MC cells were transfected with GSK3β siRNA, followed by NVP-BEZ235 treatment. The cell cycle distribution was measured by flow cytometric analysis.
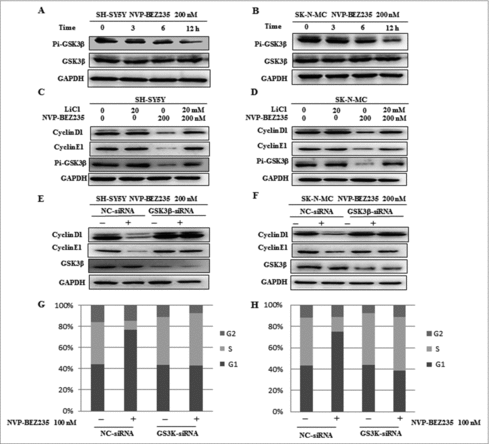
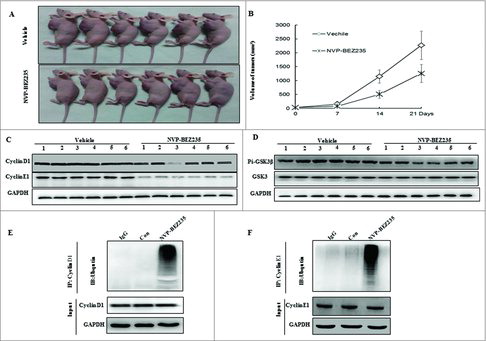
Figure 6. NVP-BEZ235 induced cyclin D1 and cyclin E1 ubiquitin-dependent proteasomal degradation through reducing p-GSK3β in NB xenograft tumor. (A) Treatment was initiated when the average tumor size reached 50 mm3. Nude Mice with SH-SY5Y xenografts were administered vehicle or NVP-BEZ235 (20 mg/kg/day). After 3-week consecutive treatment, the mice were sacrificed. (B) Tumor volumes were measured every week. (C and D) Tumor extracts were lysed and analyzed by immunoblotting against cyclin D1, cyclin E1, p-GSK3β and GSK3β. (E and F) Tumor extracts were immunoprecipitated (IP) by anti-cyclin D1 and cyclin E1 followed by immunoblotting with anti-Ub (top) and anti-cyclin D1 and cyclin E1 (bottom).