Figures & data
Figure 1. The network structure of p53 response (A) and the network structure of DNA damaged repaired (B) when SSB and DSB exist simultaneously.
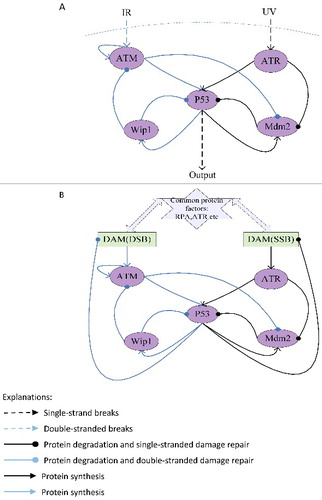
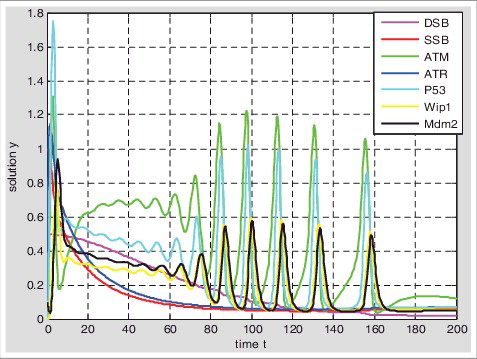
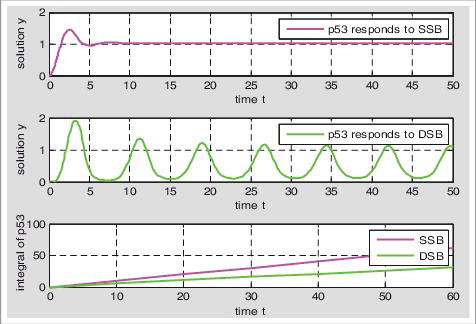
Table 1. Hybrid Model I: the numerical model of p53 response when single-stranded breaks and double-stranded breaks exist simultaneously; Hybrid Model II: the numerical model of DNA damage repair when single-stranded breaks and double-stranded breaks exist simultaneously.
Table 2. Parameters and Values for the models.
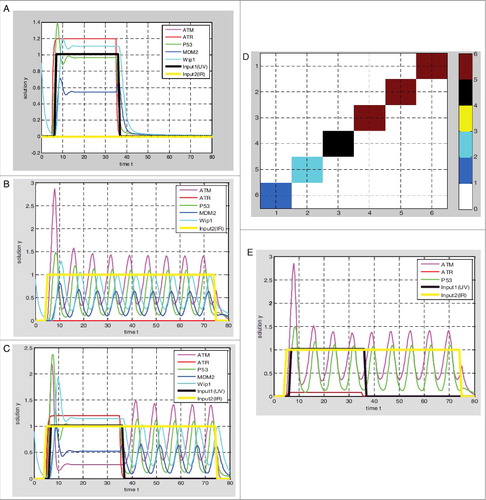
Figure 2. Simulations of p53 response based on hybrid Model I. (A) UV = 1, IR = 0, graded response of p53; (B) UV = 0, IR = 1, the digital impulse response of p53; (C) UV = 1, IR = 1, p53 preferentially responds to DNA single-stranded breaks; (D) the dark red block represents the graded response of the p53, the blue block represents the digital impulse response of p53, the green block and the black block represent the transition state, the ATR protein production rate decreasing from 3 to 0, when the ATR production rate is reduced to 0–0.5, the p53 responds to double-stranded breaks firstly, and in the range of 1.5–3 p53 responds to single-stranded breaks firstly; (E) when ATR production rates are 0.2, p53 responds to DNA double-stranded breaks firstly.