Figures & data
Figure 1. Depletion of HEATR1 stabilizes p53 A. U2OS cells were transfected with control or HEATR1 siRNAs, cell lysates were prepared 72 h after transfection and immunoblotted with indicated antibodies. B. U2OS cells were transfected with control or HEATR1 siRNAs, treated with 50 μg/ml cycloheximide (CHX) 72 h later and harvested at indicated time points. Cell lysates were immunoblotted with indicated antibodies.
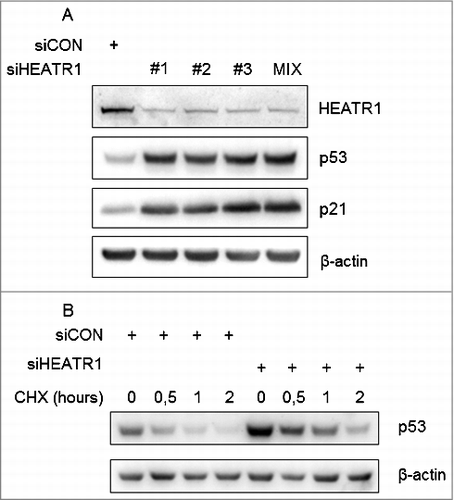
Figure 2. Knockdown of HEATR1 leads to impaired proliferation and induces p53-dependent cell cycle arrest A. U2OS cells were transfected with control or HEATR1 siRNAs and 100000 cells were seeded. Cell counts were determined at the indicated time points after transfection. Error bars represent SDs, n = 3. Significance determined by two-tailed student's t-test: * P<0,05. B. U2OS cells were transfected with the indicated siRNAs and cell cycle profiles were assessed by flow cytometry 72 h after transfection. Results are representative of three independent experiments. C. U2OS cells were transfected with the indicated siRNAs and labeled with 10 μM 5-ethynyl-2′-deoxyuridine (EdU) for 30 min. The cells were fixed and incorporated EdU was visualized by click chemistry. The nuclei were stained by DAPI. Results are representative of three independent experiments. Bar, 10 µm.
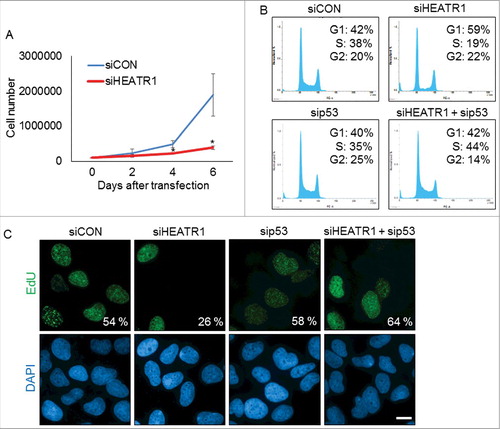
Figure 3. HEATR1 is localized to the nucleoli A. U2OS cells were fixed and immunostained with HEATR1 and nucleophosmin (NPM) antibodies. Nuclei were visualized by DAPI staining. Bar, 10 µm. B. U2OS cells were transfected with indicated siRNAs and fixed and immunostained with HEATR1 antibody 72 after transfection. Nuclei were visualized by DAPI staining. Bar, 10 µm.
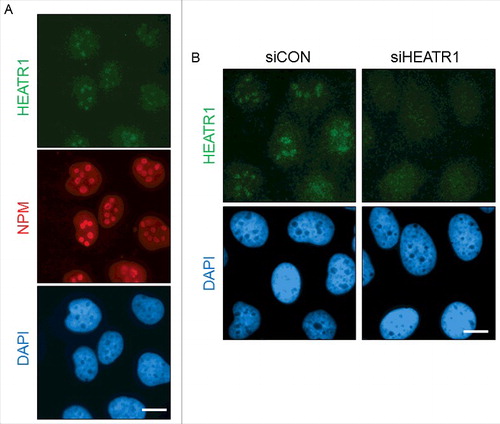
Figure 4. HEATR1 knockdown induces ribosomal stress A, B. U2OS cells were transfected with indicated siRNAs, fixed and immunostained with fibrillarin (FBL), nucleophosmin (NPM), nucleolin (NCL) and p53 antibodies 72 h after transfection. Nuclei were visualized with DAPI staining. Bar, 10 µm. C. U2OS cells were transfected with indicated siRNA, lysed and immunoblotted with indicated antibodies 72 h after transfection. D. U2OS cells were transfected with control or HEATR1 siRNAs. Cell lysates were immunoprecipitated 72 h after transfection with control (IgG) or RPL5 antibodies and immunoblotted with indicated antibodies.
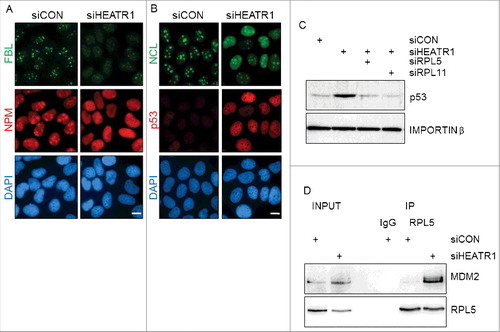
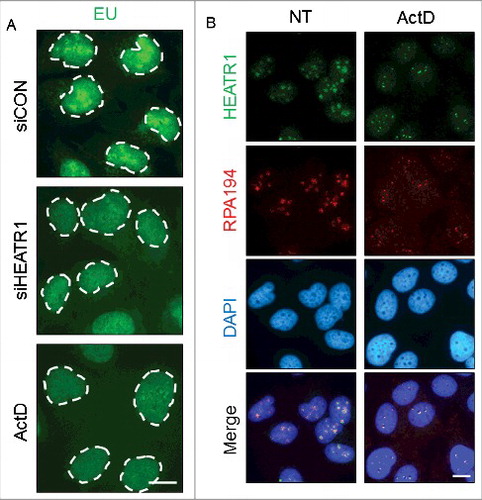
Figure 5. HEATR1 is involved in rRNA synthesis. A. U2OS cells were transfected with indicated siRNAs or treated with 5 nM ActD overnight and labeled with 1 mM 5-ethynyl uridine (EU) for 30 min. EU detection was performed by click chemistry. Bar, 10 µm. B. U2OS cells were mock- or actinomycin D- (ActD, 5nM) treated overnight, then fixed and immunostained with indicated antibodies. Nuclei were visualized by DAPI staining. Bar, 10 µm.