Figures & data
Figure 1. Interaction between Chk1 and MYPT1. (A) Chk1 was immunoprecipitated from HeLa cells, and samples were analyzed by SDS-PAGE with Coomassie blue staining. Chk1-interacting proteins were identified by mass spectrometry. Shown in the table are the proteins identified in the immunocomplex. The number of peptides and peptide coverage pertentage were indicated. (B) Chk1 immunoprecipitates were blotted with anti-Chk1 and anti-MYPT1 antibodies.(C) HeLa cells transfected with vector or HA-MYPT1, and Flag-Chk1 constructs were subjected to IP and IB assays using the antibodies indicated. Asterisks indicate IgG. (D) Domain structures of MYPT1. The PP1 binding motif, the ankyrin repeats, and the Leucine zipper domain, and the boundaries of MYPT1 fragments used in this study are indicated. (E) Bacterially expressed MYPT1 fragments were incubated with lysates from HeLa cells transfected with HA-Chk1. Asterisks indicate the corresponding bands.
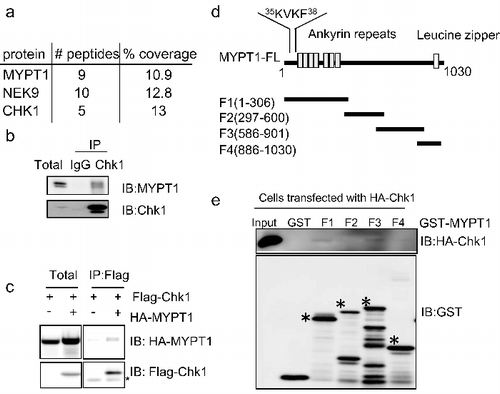
Figure 2. Ser20 is identified as one of the Chk1-dependent phosphorylation sites. (A) Recombinant Chk1 and MYPT1 were incubated in kinase buffers containing 32P-ATP, and an IVK assay was carried out. Coomassie blue staining showed input MYPT1 proteins and autoradiography showed phosphorylated GST-MYPT1. (B) LC-MS/MS analysis identified Ser20 of MYPT1 as one of the sites phosphorylated by Chk1 in vitro. From this collision-induced dissociation spectrum, a phosphorylated peptide WIG(pS)ETDLEPPVVK of MYPT1 was identified following incubation with Chk1 in an IVK reaction. “b” and “y” ion series represent fragment ions containing the N- and C-termini of the peptide, respectively. (C-D) S20A mutant was constructed in GST-MYPT1-FL (C) and -F1 (D) fragment, and IVK assays were carried out using WT and S20A of GST-MYPT1-FL or F1 proteins. (E) A comparison between Chk1 phosphorylation consensus sites and the adjacent amino acids of MYPT1 Ser20.
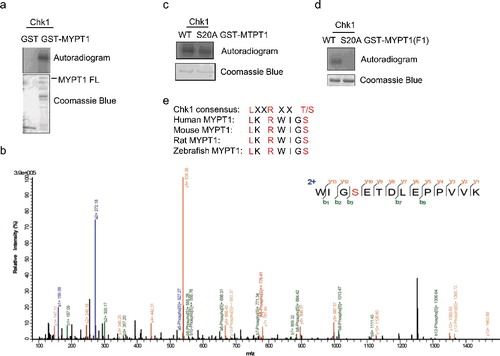
Figure 3. Ser20 of MYPT1 is phosphorylate by Chk1. (A) HeLa cells were transfected with Flag-Chk1 or Flag-Chk1-kinase dead (KD), and then IPed with anti-Flag antibodies. The immunoprecipitates were used in an IP-kinase assay using recombinant GST-MYPT1 as substrates. The reaction was then terminated and blotted with antibodies indicated. (B) IVK assays using recombinant His-Chk1 and GST-MYPT1 or GST-MYPT1-S20A as substrates. The products were then blotted with pS20 antibodies. (C) Ser20 of MYPT1 was phosphorylated in vivo. HeLa cells transfected with HA-MYPT1 were treated with Noc, Noc plus UV, Noc plus UV plus UCN-01 (an inhibitor of Chk1). Lysates from these cells were blotted with pS20 antibodies.
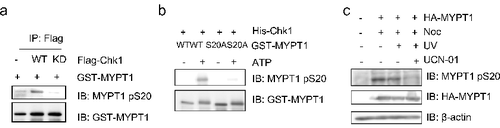
Figure 4. pS20 promotes the degradation of MYPT1. (A) HeLa cells transfected with Flag-Chk1 and HA-Ub were treated with MG132 or left untreated, IPed with anti-MYPT1 antibodies and IBed with the antibodies indicated. (B) Cells were transfected with vector, WT or KD of Flag-Chk1 and HA-Ub plasmids, then analyzed as in (A). (C) Cells were treated with UCN-01 or left untreated, then analyzed as in A. (D) Cells were transfected with HA-MYPT1, treated with UCN-01 or left untreated, then incubated with CHX for the time indicated. (E) Quantitation of the results in (D). (F) Cells were transfected with WT or S20A of HA-MYPT1, then incubated with CHX. (G) Quantitation of the results in (F).
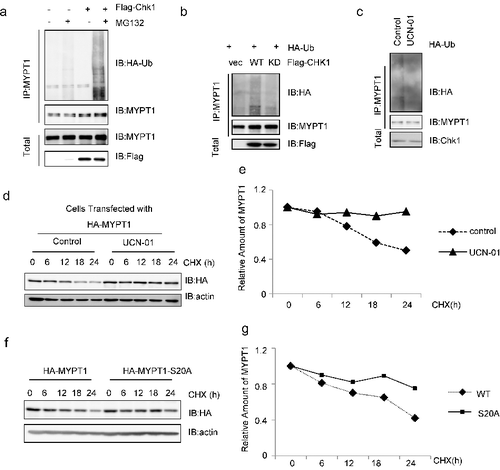
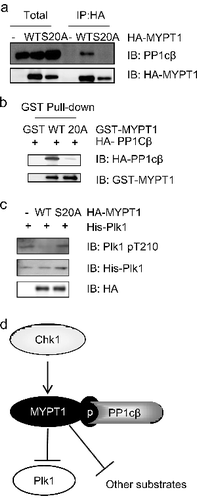
Figure 5. pS20 is essential for the interaction between MYPT1 and PP1cβ. (A) HeLa cells were transfected with HA-MYPT1-WT or S20A plasmids, then subject to IP and IB analysis as indicated. (B) Recombinant GST-MYPT1 and S20A proteins were used to pulldown transfected HA- PP1cβ. (C) HeLa cells were transfected with HA-MYPT1-WT or S20A plasmids and treated with Noc, then subject to IP with anti-HA antibodies, followed by IP-phosphatase assays using active Plk1 as substrates. The products were then blotted with anti-Plk1-pT210 antibodies. (D) A proposed model of how Chk1 inhibits Plk1 through MYPT1. Chk1 phosphorylates MYPT1 at Ser20 to promote the interaction between MYPT1 and PP1cβ. MYPT1 then targets PP1cβ to Plk1 to dephosphorylate Plk1 at pT210, and possibly other proteins.