Figures & data
Figure 1. Surgical orthotopic implantation (SOI). A. An approximately 10 mm incision was performed at the upper portion of the abdomen (black dotted line). B. The stomach was gently exteriorized from the peritoneum. C. The serosa of the anterior gastric wall was carefully incised in order to not make a perforation (white arrow). D. A small fragment of GIST was implanted at the incised site on the gastric wall (white arrow).
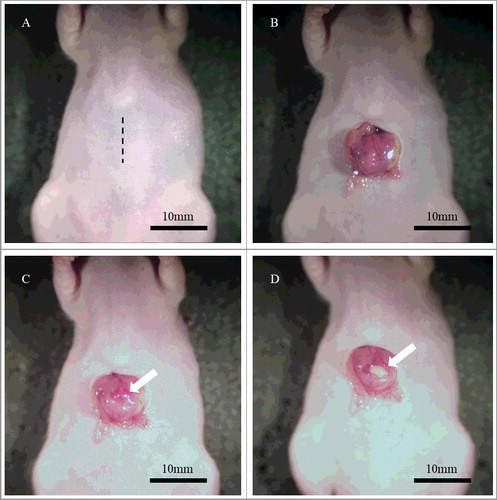
Figure 2. Treatment protocol. G1: untreated group; G2: imatinib (50 mg/kg, p.o., daily, 3 weeks); G3: sunitinib (40 mg/kg, p.o., daily, 3 weeks); G4: regorafenib (30 mg/kg, p.o., daily, 3 weeks); G5: pazopanib (100 mg/kg, p.o., daily, 3 weeks). Each group consisted of 6 mice. All mice were sacrificed on day 22.
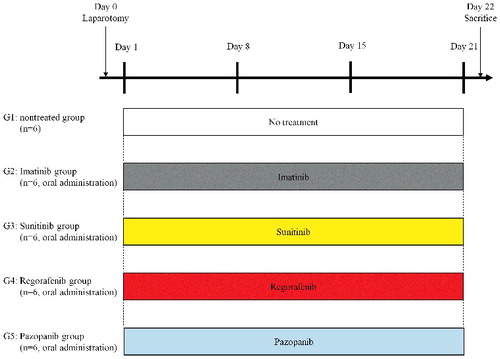
Figure 3. Tumor volume ratio. Bar graphs show the PDOX tumor volume ratio (post-treatment volume / pre-treatment volume) on day 0 and 22. Regorafenib regressed tumor growth significantly compared to the untreated group (P < 0.001). There was also a significant difference between the untreated group and sunitinib (P = 0.002). Imatinib and pazopanib did not show significant efficacy (P = 0.886 and P = 0.766) respectively compared to the untreated control. Error bars: ± SD.
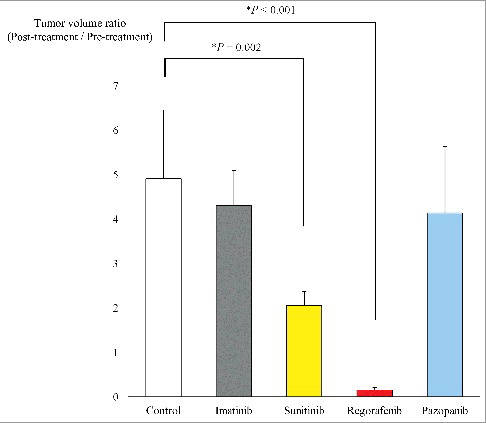
Figure 4. Body weight of each group. Bar graphs shows body weight of the GIST PDOX mice treated with each drug. There was no significant difference between any group. Error bars: ± SD.
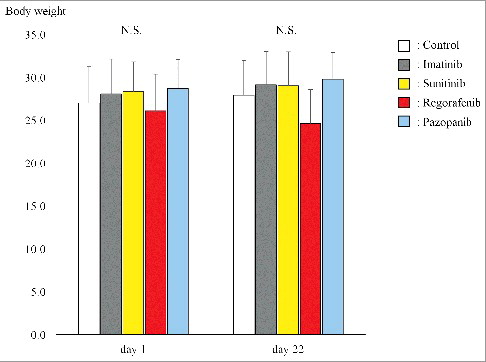
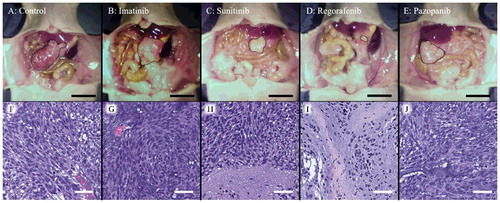
Figure 5. A-E. Representative macroscopic images of the GIST PDOX model. Representative images of the GIST PDOX on day 22. The area surrounded by the black broken line is tumor. Scale bar is 10 mm. F-J. Hematoxylin and eosin (H&E) staining of a representative tumor from each group. Sunitinib and regorafenib showed necrosis (H and I). Scale bar is 50 µm.