Figures & data
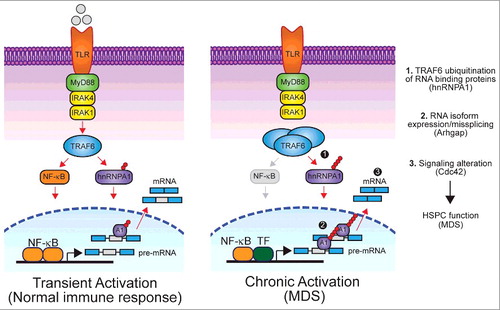
Figure 1. Overview of isoform usage in response to transient (left) or chronic TLR signaling (right). During a normal immune response, TRAF6 transiently catalyzes K63-linked ubiquitin (Ub) chains on RNA binding proteins, like hnRNPA1, which leads to differential splicing of target genes, such as Arhgap, and an appropriate balance of isoform expression. Chronic TLR activation results in TRAF6 overexpression and continuous ubiquitination of hnRNPA1 (1), which leads to skewed isoform usage of Arhgap (2) and subsequent alteration of downstream signaling pathways (3), such as the one involving the small G-protein GTPase Cdc42. Overactive Cdc42 signaling contributes to HSPC defects and myelodysplasia associated with bone marrow failure. Transcription of alternatively spliced genes was not exclusively driven by NF-κB and likely involves other transcription factors (TF). NF-κB signaling was not hyperactive following TRAF6 overexpression (indicated in grey).