Figures & data
Figure 1. Compounds identified in silico activate p53-dependent GFP reporter in cells. (a) An X-ray structure of a typical Isatin Mannich Schiff base derivative (IMSBD = Au, Av, Ap, Ar, and d) compound (scale is 17 Å) that has topological and lipophylic characteristics similar to the alpha-helical part of p53 TAD (grey ribbon) that binds MDM2. (b) Schematic diagram explains how small molecules, by dissociating the p53-Mdm2 interaction, liberate p53 that binds the DNA response elements and activates transcription of GFP; (c) representative images of U2-OS-pLV1 cell line responses to treatment with Nutlin-3A and isatin-based compounds (Au, Av, Ap, Ar, and D) by activating GFP expression; (d) To investigate effects of ISMBDs on the p53-Mdm2 interaction, the recombinant purified GST-p531-40 and 6-HIS-Mdm21-104 proteins were incubated in the presence of ISMBDs, precipitated on glutathione beads and, following washes, analyzed by western blotting for the p53-bound Mdm2 protein; (e) Western blot signals of Mdm2 used in reactions (Input), p53-bound Mdm2, and the levels of GST-p53 used in the assay are shown.
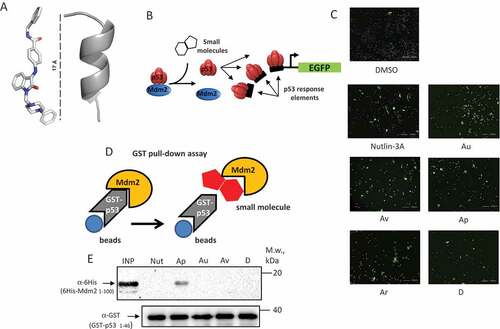
Table 1. Activation of p53-reporter and cytotoxicity upon treatment of U2-OS cells with ISMBD compounds.
Figure 2. ISMBDs augment expression of the PUMA-Luc reporter in p53-dependent manner. (a) HCT116 (p53+ and p53-) cells were cotransfected with the PUMA-luc reporter vector and pRL Renilla Luciferase Control Reporter Vector as an internal control for transfection efficiency. Transfected cells were treated with ISMBDs (Au, Av, Ap, Ar, and D) and Nutlin-3A (positive control) to measure luciferase activity [Citation53]; (b) Western Blot analysis of p53 protein levels was performed with the same lysates, which were used for Luciferase assay. Actin was used as a loading control.
![Figure 2. ISMBDs augment expression of the PUMA-Luc reporter in p53-dependent manner. (a) HCT116 (p53+ and p53-) cells were cotransfected with the PUMA-luc reporter vector and pRL Renilla Luciferase Control Reporter Vector as an internal control for transfection efficiency. Transfected cells were treated with ISMBDs (Au, Av, Ap, Ar, and D) and Nutlin-3A (positive control) to measure luciferase activity [Citation53]; (b) Western Blot analysis of p53 protein levels was performed with the same lysates, which were used for Luciferase assay. Actin was used as a loading control.](/cms/asset/e39eee77-a1f1-4720-87bc-5db10844f966/kccy_a_1506664_f0002_oc.jpg)
Figure 3. ISMBDs differentially affect expression of p53 target genes. U2-OS (p53+ and p53-) cells treated with ISMBDs (Au, Av, Ap, Ar, and D) were subjected to RT-PCR analysis to measure expression levels of BAX (a), TP53 (b), P21 (c), MDM2 (d) and PUMA (e) genes. Signals of non-treated p53- samples were arbitrary set as 1. Anova statistical significance values are denoted as follows: * p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001, **** p ≤ 0.0001. (3F) The corresponding samples were also examined by western blotting using antibodies against p53, Mdm2, Puma, and actin (loading control).
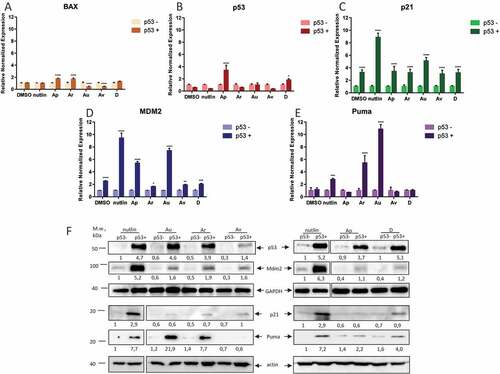
Figure 4. ISMBDs decrease cell viability of HCT116 and U2-OS cells in p53-dependent manner. HCT116 (p53+ and p53-) (a) and U2-OS (p53+ and p53-) cells expressing pLV1-GFP reporter (b) were treated with the indicated concentrations of ISMBDs (Au, Av, Ap, Ar, and D) and counted for the number of cells using the automated microscopy system Operetta. The initial number of cells before treatment in each case was arbitrary set as 100%.
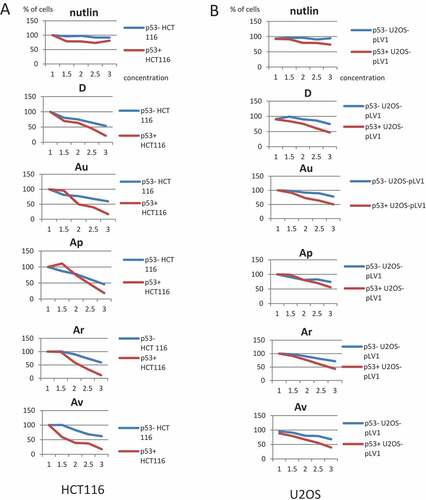
Figure 5. ISMBDs are non-genotoxic and attenuate proliferation of cancer cells in p53-dependent manner. U2-OS p53+ (a) and p53- (b) cells were treated with ISMBDs (Au, Av, Ap, Ar, D, An and 2), or doxorubicin (positive control) following immunostaining with antibody against γ–H2Ax. The number of nuclear foci was calculated in each case using the automated microscopy system Operetta to assess the levels of DNA damage. (c) HCT116 (p53+ and p53-) cells were treated with compounds mentioned above and examined for their proliferation potential by colony formation assay. Experiments were done in triplicates. After two weeks incubation cells were fixed, stained and counted in each case. (d) To evaluate p53-specific effects of ISMBDs on proliferation, percentage ratios of colonies formed in p53+ versus p53- cells were calculated. Anova statistical significance values are denoted as follows: * p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001, **** p ≤ 0.0001.
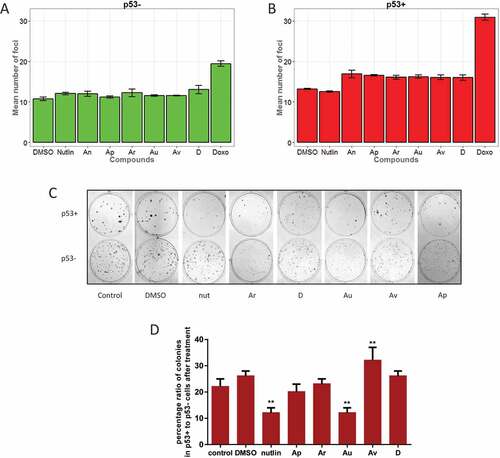
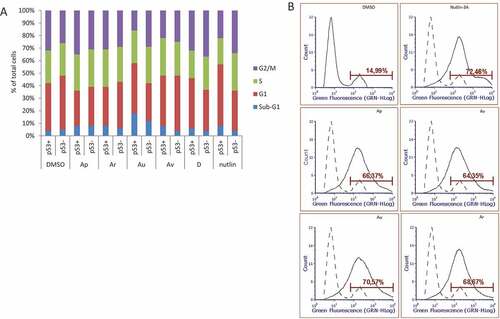
Figure 6. ISMBDs promote p53-dependent cell death and arrest of proliferation. (a) U2-OS (p53+ and p53-) cells treated with ISMBDs (Au, Av, Ap, Ar, and D) and Nutlin-3A (positive control) were stained with propidium iodide (PI) and analysed by FACS. Cell death was calculated by estimating the number of cells in sub-G1 phase. (b) For measuring the effect of compounds on apoptosis, cells were treated with 3 μM of ISMBDs following the staining with Alexa Fluor 488 Annexin V Kit and flow cytometry. Percentages of Annexin V-positive cells after treatment are indicated.