Figures & data
Figure 1. Flow cytometry screening of CK14+cells. The model control group (A). hESCs cultured in medium without RA, BMP4, and AA have low CK14 expression (B). hESCs induced by RA, BMP4, and AA have high CK14 expression (C).
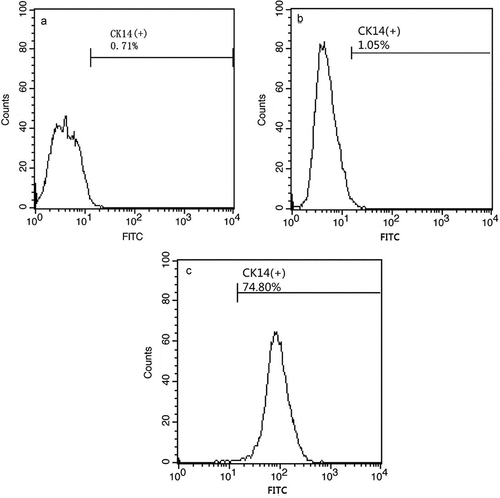
Figure 2. Morphology of cultured H9 human embryonic stem cells. hESCs induced by RA, BMP4, and AA appear similar to keratinocytes (B), cells cultured without RA, BMP4, and AA are fusiform in appearance (A).
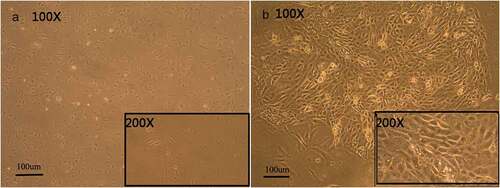
Figure 3. Hematoxylin–eosin staining of EKCs and biological scaffolds shows the in vitro structural organization. In A1, the cell nucleus is not clearly visible and a paving-stone morphology is not apparent. In A2, the number of adherent cells is decrease, nuclei are not apparent, and a paving-stone morphology is absent. A3 shows that cells of uniform size have adhered to the biological scaffold in a polygonal array that has a paving-stone appearance. Panel B shows the biological scaffold without any adherent cells.
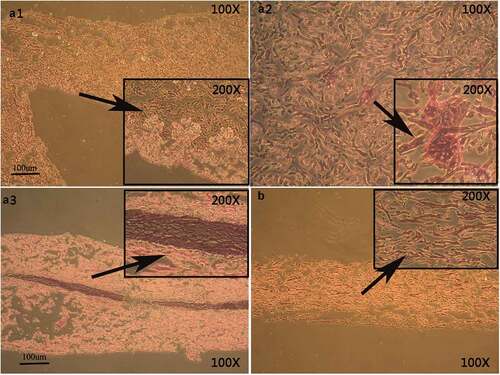
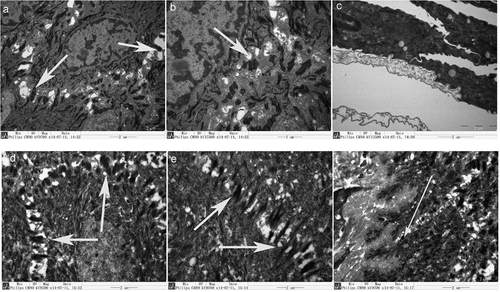
Figure 4. Immunohistochemical staining shows CK14 expression of CK14+EKCs in close contact with the biological scaffold (A, B) and with an arrangement similar to the basal cell layer of normal oral mucosal epithelium (C, D).
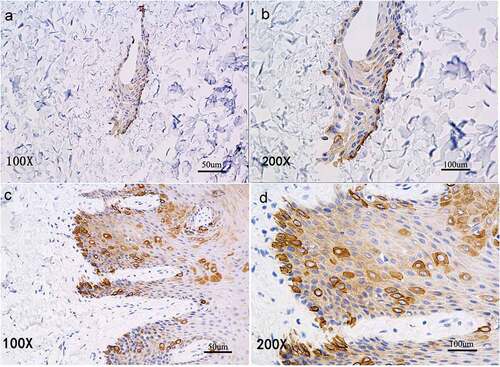
Figure 5. Immunohistochemical staining of P63 expression. P63+cells are scattered within each layer of the in vitro reconstructed tissue (A, B) and distributed in a pattern similar to that in normal oral mucosal epithelium (C, D).
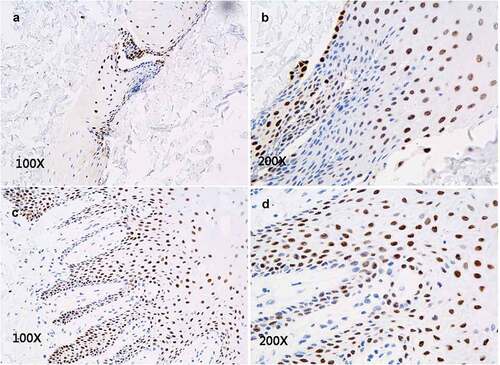
Figure 6. Immunofluorescence staining of CK15 expression in epithelial tissue shows CK15+cells (green) scattered within every layer of in vitro reconstructed tissue (A, B) and with distribution pattern similar that seen in normal oral mucosal epithelium (C, D).
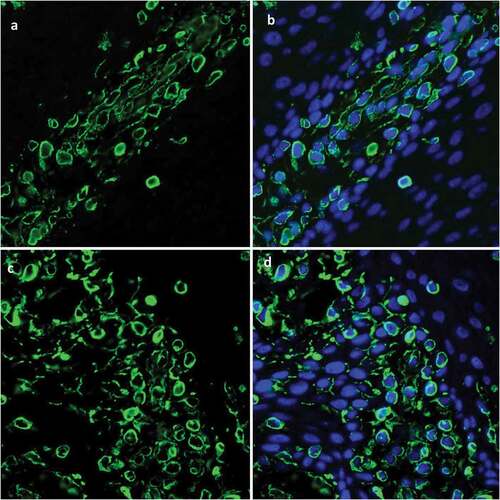
Figure 7. Scanning electron micrographs show intercellular junctions. In the reconstructed tissue, many desmosomes formed between cells (A, B), but no half-desmosomes formed between the cells and the basal layer (C). Desmosomes are shown between cells in normal oral mucosal epithelium (D, E), and half-desmosomes occur between cells and the basal lamina (F).