Figures & data
Figure 1. Modulation of TP53INP2 during autophagy. In basal conditions, TP53INP2 and LC3 remain mainly nuclear. Under autophagy activation by amino acid starvation or rapamycin treatment, TP53INP2 moves to the cytosol together with LC3. In the cytosol, TP53INP2 interacts with LC3, GATE16 and ATG7 to promote autophagosome formation and maturation. Under most conditions, TP53INP2 leaves the autophagosome before its fusion with lysosomes.
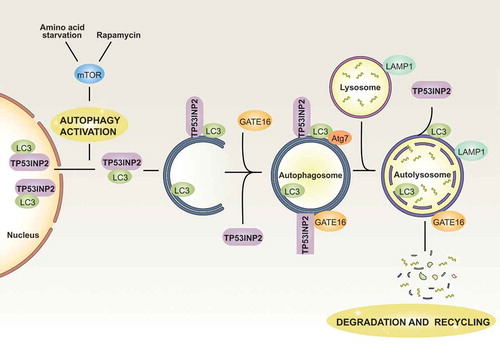
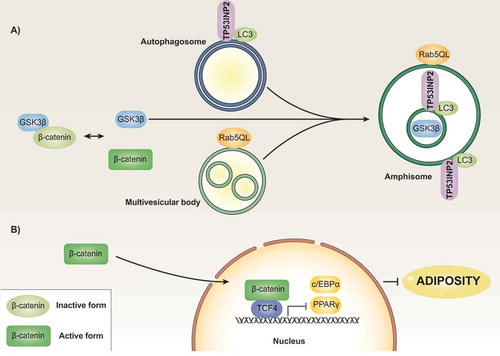
Figure 2. Model of TP53INP2 action in adipose precursor cells. Regulatory protein β-CATENIN is found in inactive and active states. The formation of a complex with GSK3β, among other proteins, leads to β-CATENIN phosphorylation followed by further proteasomal degradation. The presence of a constitutively active mutant form of Rab5 (Rab5QL) causes the formation of enlarged MVBs, which occurs in the absence of changes in the localization of GSK3β. Under these conditions, TP53INP2 promotes the fusion of autophagosomes with MVBs in such a way that GSK3β is internalized into the MVB/amphisome compartment in an ESCRT-dependent manner (panel A). This process leads to activation of β-CATENIN, which blocks the transcription of genes involved in the induction of the adipogenic program (panel B).