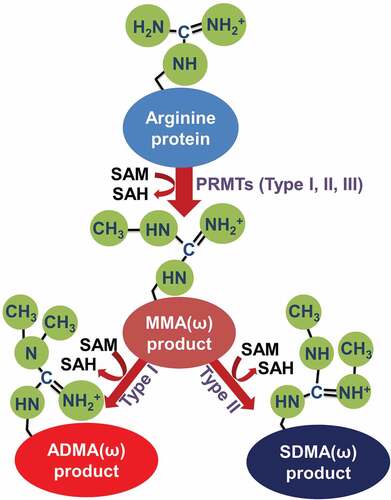
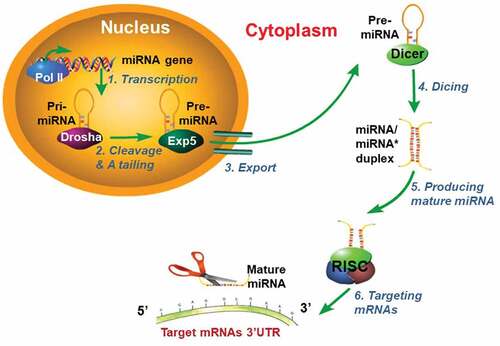
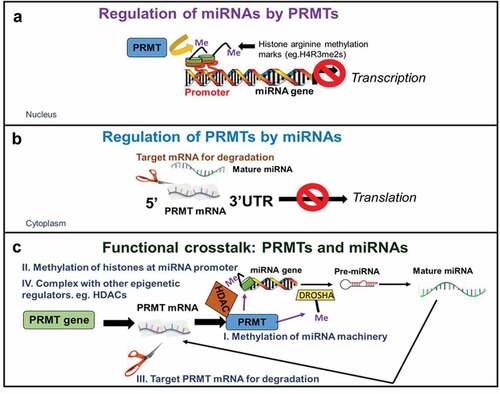
Figures & data
Table 1. List of known interplay between PRMTs and miRNAs in cancer.
Table 2. List of known interplay between PRMTs and miRNAs in other conditions.