Figures & data
Figure 1. Mammalian oocyte maturation. Oocytes are arrested at prophase I, also known as germinal vesicle (GV) stage. Upon hormonal stimulation, fully-grown oocytes resume meiosis with germinal vesicle breakdown (GVBD). Chromosomes (in blue) condense, the bipolar spindle forms and microtubules attach to the chromosomes throughout prometaphase. In metaphase I chromosomes that are properly attached to the spindle and aligned at the metaphase plate migrate to the cortex. Chromosomes segregate during anaphase I with the extrusion of a first polar body. Oocytes immediately enter meiosis II and arrest at metaphase II awaiting fertilization.
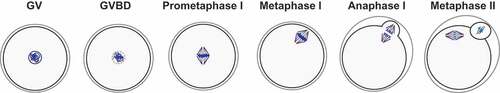
Figure 2. Regulation of cyclin B1 degradation during meiosis I. At early prometaphase, different pools of cyclin B1 are present in the oocyte: free cyclin B1 and cyclin B1 bound to Cdk1. During prometaphase, free cyclin B1 starts to be degraded while the SAC is on, whereas Cdk1-bound cyclin B1 is protected from APC/C-dependent ubiquitination and degradation through its binding. Most of the free cyclin B1 is degraded once oocytes progress into metaphase I, while bound cyclin B1 begins to be degraded only once the SAC is satisfied and Cdc20-APC/C under SAC control becomes active. Once the APC/C is fully active, cyclin B1 bound to Cdk1 is degraded leading to a sharp decrease of Cdk1 activity and anaphase I onset.
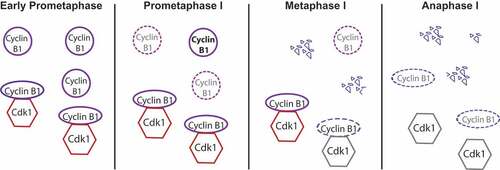
Figure 3. Protein levels of cyclin B1 vs. cyclin B2 in meiosis I. According to Citation64, preceding resumption of meiosis, cyclin B2 (in red) is translated at higher levels than cyclin B1 (in blue) while their degradation rates are similar. Therefore, at GV stage, cyclin B2 protein is more abundant than cyclin B1. After entry into meiosis I, protein levels of cyclin B2 remain similar with only a slight increase in translation. However, cyclin B1 is actively translated leading to an increase of protein levels following GVBD reaching its maximum at metaphase I. Cyclin B1 translation depends on cyclin B2 protein at entry into meiosis I. Hence, protein levels of both cyclins are regulated differentially and temporally in mouse oocytes.
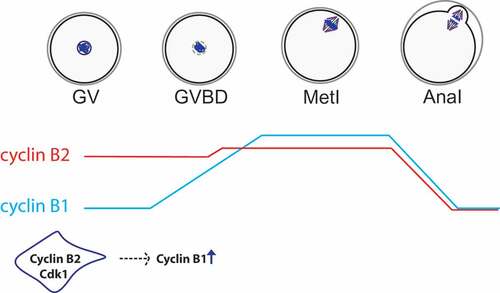
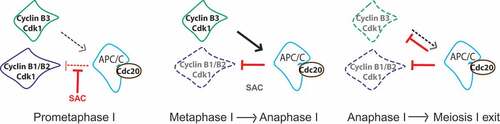
Figure 4. Model of how cyclin B3 may promote anaphase I onset in mouse oocytes. Cyclin B1/B2-Cdk1 (in blue) activity increases as oocytes progress through prometaphase into metaphase, because the APC/C (light blue) in association with its activator Cdc20 (brown) is kept in check by the SAC. At the metaphase-to-anaphase transition, the SAC is satisfied and inactivated, allowing full APC/C activity, ubiquitination and hence degradation of cyclin B1 and B2 in association with Cdk1. In oocytes, full APC/C activity requires the function of cyclin B3-Cdk1. Cyclin B3-Cdk1 (in green) promotes APC/C activity leading to chromosome segregation and exit from meiosis I. Therefore cyclin B3, a late substrate of the APC/C, shows an opposing role to cyclin B1 and B2 during mouse oocyte meiosis I.