Figures & data
Figure 1. REGγ is expressed in ApoE-/- mouse atherosclerotic plaques and induces CyPA expression. REGγ is expressed in ApoE-/- mouse atherosclerotic plaques (n = 6). (a) (arrow indicated), (b) (magnification of the boxed area in (a) and (c) (statistical analysis of relative REGγ protein expression intensity of plaque area per vascular area normalized by plaque adjacent area), REGγ was highly expressed in atherosclerotic plaques from ApoE-/-mice fed a Western diet for 12 weeks. REGγ induces CyPA expression. Knockdown of REGγ with specific shRNA or siRNA transfection, or knockout of REGγ in mice, significantly decreased CyPA mRNA expression by real time-qPCR (d and e) and RT-sqPCR (f and g) detection in indicated cells and mouse tissues. Knockdown or knockout of REGγ significantly decreased CyPA protein expression in the indicated cells and mouse tissues (h and i). Overexpression of REGγ with Flag- REGγ plasmid transfection dramatically increased CyPA expression by real time-qPCR (j), RT-sqPCR (k) and Immunoblotting (l) detection in indicated cells. Data were presented as mean ± SD of 3 independent experiments. ***,p < 0.001 (two-tailed Student’s t-test).
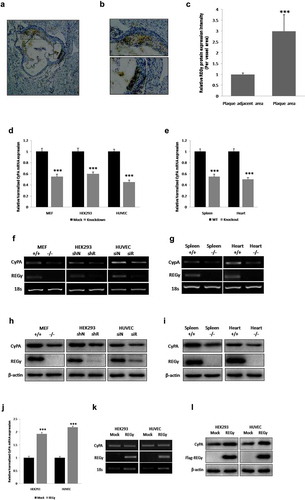
Figure 2. FoxO1 promotes CyPA expression. FoxO1 knockdown by using siFoxO1 significantly decreased CyPA mRNA expression, as determined by performing (a) real time-qPCR and CyPA mRNA expression was significantly upregulated in cells expressing WT FoxO1 and constitutively active FoxO1 A3, but not in cells expressing loss-of-function FoxO1 ∆DB, as determined by performing (b) real time-qPCR and real time-qPCR results were confirmed by performing corresponding (c and d) RT-sqPCR, which were consistent with the real time-qPCR shown. (e and f) FoxO1 knockdown with siFoxO1 significantly decreased CyPA protein expression in the indicated cells. (g and h) CyPA protein expression was significantly upregulated in cells expressing the constitutively active FoxO1 A3. Data were presented as mean ± SD of 3 independent experiments. ***,p < 0.001 (two-tailed Student’s t-test).
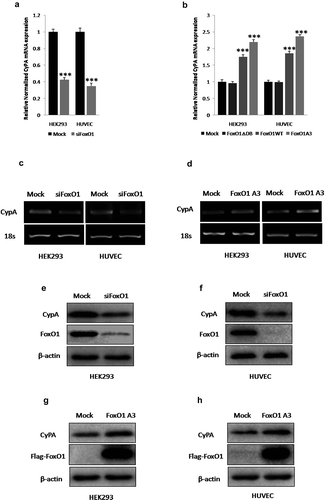
Figure 3. FoxO1 stimulates the transcriptional activity of and binds to a specific region containing the FHRE in the CyPA promoter. FoxO1 promotes the transcriptional activity of the CyPA promoter. (a and b) FoxO1 inhibition or knockdown by using FSK or siFoxO1, respectively, significantly decreased the basal transcriptional activity of the CyPA promoter, as assessed by performing the reporter gene assay by using the upstream 2,000-bp fragment of the human CyPA gene. (c) Overexpression of WT FoxO1 and constitutively active FoxO1 A3, but not of loss-of-function FoxO1 ∆DB increased the basal transcriptional activity of the CyPA promoter.FoxO1 binds to the specific region in the CyPA promoter. (d) The results of the ChIP assay showed that FoxO1 associated with the CyPA promoter at a region containing the FHRE that was 2,000-bp upstream of the transcription start site. (d) However, no binding was observed when the GAPDH promoter off-site control primers or negative antibody control IgG antibody was used. Data were presented as mean ± SD of 3 independent experiments. ***,p < 0.001 (two-tailed Student’s t-test).
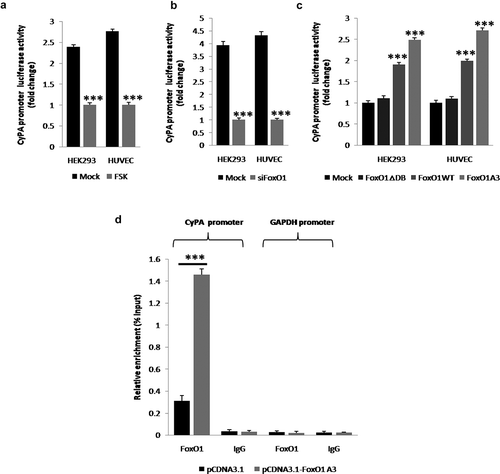
Figure 4. PKA inhibites CyPA expression. PKA inhibition or knockdown by treatment with H89, or transfection with siPKAcα, respectively, significantly increased CyPA mRNA expression according to the results of real time-qPCR (a and b). CyPA mRNA expression was significantly downregulated in cells treated with PKA activator FSK in a time-dependent manner or expressing PKAcα, as indicated by the results of real time-qPCR (c and d). RT-sqPCR was performed to confirm the results of real time-qPCR (E, F, G and H), with VCAM-1, which is regulated by PKA, as the positive control. Immunoblotting was performed to confirm the results of mRNA expression measurements, with VCAM-1, which is regulated by PKA, as the positive control. PKA inhibition or knockdown by treatment with H89 or transfection with siPKAcα, respectively, significantly increased CyPA protein expression (i and j). CyPA protein expression was significantly downregulated in cells treated with PKA activator FSK in a concentration-dependent manner or expressing PKAcα (K and L). Data were presented as mean ± SD of 3 independent experiments. **,p < 0.01, and ***,p < 0.001 (two-tailed Student’s t-test).
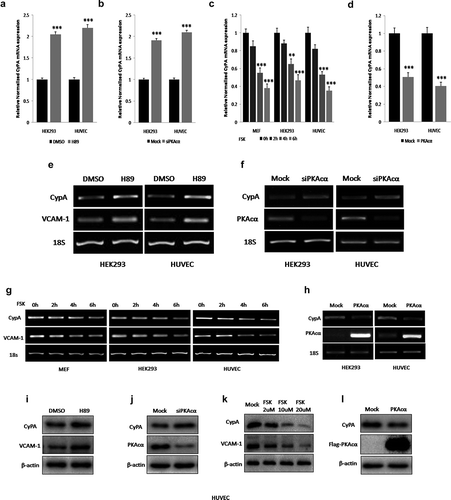
Figure 5. PKA inhibites CyPA expression via FoxO1 pathway. PKA inhibition or knockdown by treatment with H89, or transfection with siPKAcα, respectively, significantly increased CyPA mRNA expression, but this PKAcα-derived increase was diminished when cells were knocked down FoxO1 by specific siRNA transfection according to the results of real time-qPCR (a and b). CyPA mRNA expression was significantly downregulated in cells treated with PKA activator FSK or expressing PKAcα, but this PKAcα-derived decrease was rescued when cells were overexpressed WT FoxO1 and constitutively active FoxO1 A3, and the FoxO1 A3 group rescued more compared to the WT group, but not in cells overexpressed loss-of-function FoxO1 ∆DB, as indicated by the results of real time-qPCR (c and d). Immunoblotting was performed to confirm the results of mRNA expression measurements, with VCAM-1, which is regulated by PKA, as the positive control. PKA inhibition or knockdown by treatment with H89, or transfection with siPKAcα, respectively, significantly decreased FoxO1 phosphorylation at Ser256 as previously we had indentified, and associated with significant increase of CyPA protein expression, but this PKAcα-derived CyPA increase was diminished when cells were knocked down FoxO1 by specific siRNA transfection according to the results of Immunoblotting (E and F). FoxO1 phosphorylation at Ser256 was significantly upregulated as previously we had demonstrated, and associated with significant downregulation of CyPA protein expression in cells treated with PKA activator FSK or expressing PKAcα, but this PKAcα-derived CyPA decrease was rescued when cells were overexpressed constitutively active FoxO1 A3 by plasmid transfection as indicated by the results of Immunoblotting (g and h), indicating that PKA mediated CyPA protein expression partly via regulated FoxO1 phosphorylation and transcriptional activity. Data were presented as mean ± SD of 3 independent experiments. **, p < 0.01,***,p < 0.001 (one-way ANOVA with post hoc Tukey test).
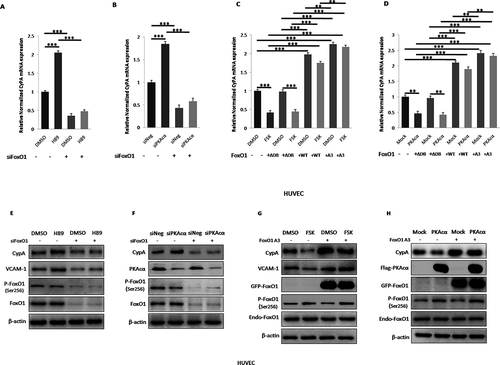
Figure 6. REGγ induces CyPA expression via the PKA-FoxO1 axis. Treatment with the PKA activator FSK significantly decreased CyPA mRNA expression in REGγ WT cells (MEF+/+ and HUVEC siN), but this decrease was not observed in REGγ knockout or knockdown cells (MEF-/- and HUVEC siR) by real time-qPCR (a and b) detection in the indicated cells, suggesting that REGγ mediated CyPA expression partly via the PKA pathway. Knockdown of REGγ significantly decreased CyPA mRNA expression, but this REGγ-derived decrease was diminished or rescued in cells after treated with the PKA activator FSK, knocked down or overexpressed PKA, knocked down FoxO1 or overexpressed FoxO1 WT and constitutively active FoxO1 A3 (the FoxO1 A3 group rescued more compared to the WT group), but not in cells overexpressed loss-of-function FoxO1 ∆DB, with real time-qPCR (c and d) detection in the indicated cells, indicating that REGγ mediated CyPA mRNA expression indeed partly via the PKA-FoxO1 pathway. Knockdown of REGγ significantly increased PKAcα protein level and FoxO1 phosphorylation at Ser256 as previously we had identified, and associated with significant decrease of CyPA protein expression, but the REGγ-derived CyPA decrease was diminished or rescued after treated with the PKA activator FSK, overexpressed PKAcα, knocked down FoxO1 or overexpressed constitutively active FoxO1 A3 by specific siRNA, or indicated plasmids transfection as indicated by the results of Immunoblotting (e–h), indicating that REGγ mediated CyPA protein expression partly via regulated PKAcα protein stability and PKAcα-derived FoxO1 phosphorylation and transcriptional activity, depended on REGγ-PKA-FoxO1 axis. Data were presented as mean ± SD of 3 independent experiments. **, p < 0.01, ***,p < 0.001 (one-way ANOVA with post hoc Tukey test).
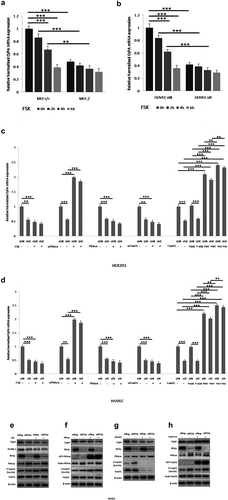
Figure 7. REGγ regulates HUVEC viability and triggers ECs apoptosis partly via CyPA activity. Cell viability and apoptosis assays were performed using HUVECs transiently pre-transfected with vector or CyPA plasmid for 24 h, followed by transfection with siNeg or siREGγ for 24 h, and treatment with CHX for 24 h. REGγ-stimulated CyPA-dependent ECs death. (a) Cell death decreased robustly in cells transfected with siREGγ and treated with CHX. However, this decrease in cell death was not observed in cells overexpressed CyPA. (b) ECs death was indentified to be caused by apoptosis with nuclei morphological staining (arrowhead), based on the analysis of nuclear morphology, by performing fluorescence microscopy, and (c) statistical analysis of apoptotic cell percentage was shown. For this, HUVECs were fixed, and apoptotic cells were identified based on the morphological staining of nuclei, as described in Materials and Methods. (d) TUNEL assays were performed to verifty the results of nuclei morphological staining, the FITC-labeled TUNEL-positive cells with green fluorescence were defined as apoptotic cells, and corresponding (e) statistical analysis of apoptotic cell percentage was shown. Data were presented as mean ± SD of 3 independent experiments. *, p < 0.05, **, p < 0.01, ***,p < 0.001 (one-way ANOVA with post hoc Tukey test).
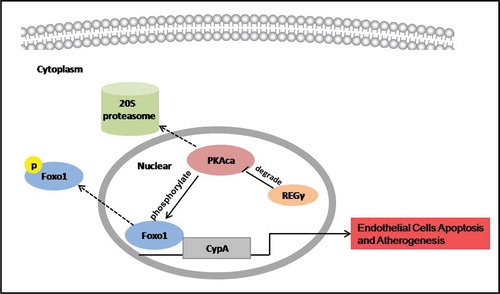
Figure 8. REGγ, via the PKA-FoxO1 axis, stimulated CyPA expression and CyPA-dependent ECs apoptosis and had functional implications in atherogenesis. REGγ activated the 20S proteasome to promote PKAcα degradation and inhibite the phosphorylation at Ser256 and nuclear export of FoxO1, thereby increasing FoxO1 nuclear accumulation and transcriptional activity. FoxO1 stimulated CyPA expression by binding to a specific region containing the forkhead-responsive element in the CyPA promoter. REGγ, via the PKA-FoxO1 axis, stimulated CyPA expression and CyPA -dependent endothelial cells apoptosis in vitro and had functional implications in atherogenesis in vivo.