Figures & data
Figure 1. RACK1 forms a complex with Aurora-A. (a) RACK1 associates with Aurora A directly in vitro. His-Aurora A was incubated with GST or GST-RACK1 for 2 h, then added glutathione-Sepharose beads and incubated for additional 1 h at 4°C. The beads were spinned down and washed with PBS for 3 times, and the bound proteins were analyzed by immunoblot. (b) RACK1 interacts with Aurora-A-Cd in vivo. 293T cells were transfected with Myc-Nt plus HA-RACK1 or Myc-Cd plus HA-RACK1 and cell lysates were analyzed by immunoprecipitation (IP) and western blotting with anti-Myc or HA antibodies, respectively. Whole-cell lysates were blotted and shown as the input. (c) RACK1 4–7 WDs interact with Aurora-A in vivo. 293T cells were transfected with Aurora-HA plus Myc-tagged RACK1 (1–4) WD, RACK1 (4–6)WD, RACK1(5–7)WD, respectively. The cell lysates were analyzed by IP against HA antibody and western blotting with anti-Myc.
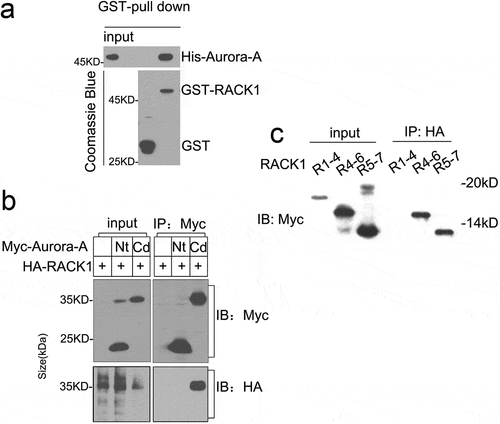
Figure 2. RACK1 co-localized with Aurora-A at centrosome. The subcellular localization of RACK1 and Aurora-A were examined by using anti- Aurora-A polyclonal antibody and anti- RACK1 monoclonal antibody in mitotic HeLa cells, respectively. Scale bar: 10 µm.
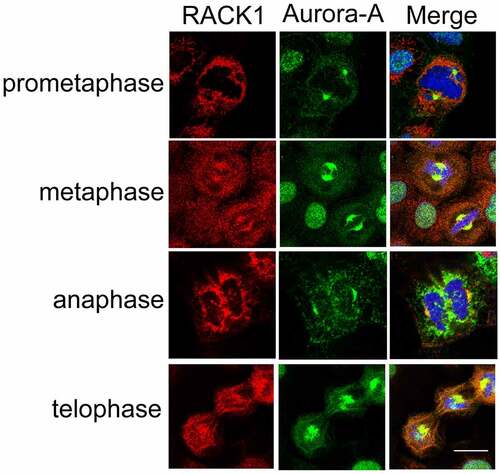
Figure 3. RACK1 regulates the kinase activity and protein stability of Aurora-A. (a) RACK1 induces the auto-phosphorylation of Aurora-A in vitro. Purified His-tag Aurora A was incubated with various concentrations of His-tag RACK1 for 30 min at 30°C. After the reaction, equal volume of 2× SDS sampling buffer was added and was boiled for 10 min. These samples were subjected to SDS-PAGE and immunoblotted by using indicated antibodies. (b) RACK1 induces the auto-phosphorylation of Aurora-A in vivo. Myc-Aurora A were co-transfected with HA vector or HA-RACK1 into HeLa cells. 48 hours later, the cells were harvested and the cell lysates were immunoprecipitated by using anti-Myc antibody, then analyzed by western blot using anti-phos-Aurora-A antibody. Whole-cell lysates were blotted using anti-HA and anti-Myc antibodies. (c) RACK1 depletion impaired the stability and the activation of Aurora A. HeLa cells treated with control siRNA or RACK1 siRNA were synchronized by using double thymidine block, and then harvested at various time points, and analyzed by western blot using indicated antibodies. (d) Myc-Aurora-A was co-transfected with gradient levels of HA-RACK1 into HeLa cells. eGFP-N1 was co-transfected as a transfection efficiency control. 48 hours later, the cells were harvested and the cell lysates were immunoblotted with anti-Myc and anti-HA antibodies. (e) Myc-Ajuba was co-transfected with wide-type Aurora-A or the kinase-inactive mutant thereof (K162R) into HeLa cells. 48 h later, the cells were harvested and the cell lysates were immunoblotted with anti-Myc antibody. (f) Lower degradation of endogenous Aurora-A with overexpression of RACK1. HeLa cells transfected with HA empty vector of HA-RACK1 were treated 20 ug/ml cycloheximide for 10, 20, 40, and 80 min. The levels of Aurora-A, endogenous RACK1, HA-RACK1 were analyzed using western blotting. Actin levels are shown as loading controls.
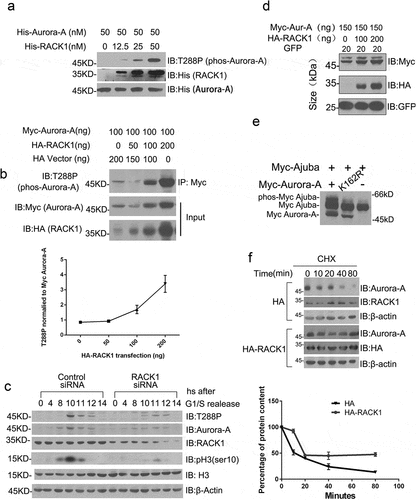
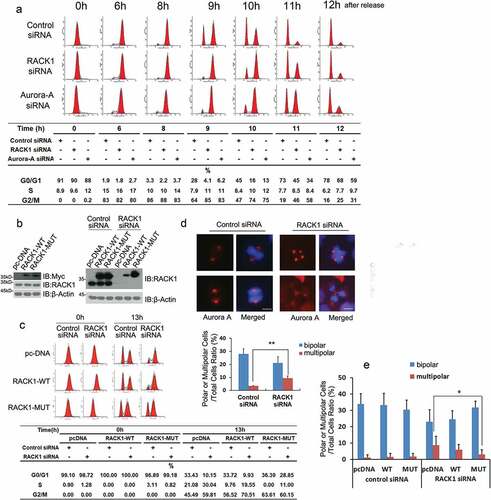
Figure 4. RACK1 is essential for mitotic entry and bipolar spindle assembly. (a) RACK1 is essential for mitotic entry. HeLa cells treated with control siRNA, RACK1 siRNA or Aurora-A siRNA were synchronized by using double thymidine block, and then harvested at various time points, and cell-cycle progression was monitored by flow cytometry using propidium iodide staining. (b) Exemplar western blot of RACK1 in the HeLa cells stably expressing pcDNA3.1, RACK-WT or RACK-MUT. Exemplar western blot of endogenous RACK1 and Myc-RACK1 in the stable lines mentioned above with treatment of either control siRNA or RACK1 siRNA. (c) The HeLa cells stably expressing pcDNA3.1, RACK-WT or RACK-MUT were synchronized and then transfected with either control siRNA or RACK1 siRNA in the interval between the two thymidine blocks.13hr after the release, half of the cells were analyzed by western blot using the RACK1 antibody. Another half of the cells were analyzed by flow cytometry. (d) RACK1 is essential for bipolar spindle assembly. Hela cells treated with control siRNA or RACK1 siRNA were synchronized by using double thymidine block, and then analyzed by Immunofluorescence staining using indicated antibodies. The percentages of cells with abnormal mitotic spindles were quantified. Scale bar: 10 µm. (e) The overexpression of RACK1 can rescue the defect of abnormal spindle assembly. The HeLa cells stably expressing pcDNA3.1, RACK-WT or RACK-MUT were synchronized and then transfected with either control siRNA or RACK1 siRNA in the interval between the two thymidine blocks. The percentages of cells with abnormal mitotic spindles were quantified after immunofluorescence staining.