Figures & data
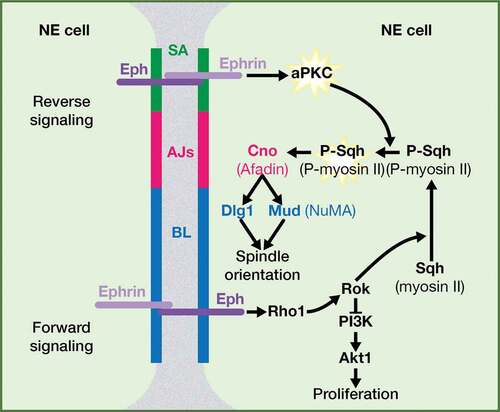
Figure 1. Ephrin and Eph receptor family members in vertebrates and in Drosophila. The structure of Eph receptors is similar in vertebrates and in Drosophila, while Drosophila Ephrin is more similar to vertebrate ephrinBs; PDZ BD: PSD95/Dlg1/ZO-1 Binding Domain; RBD: Receptor Binding Domain; LBD: Ligand Binding Domain; CRD: Cysteine-rich domain; FNIII: Fibronectin type III; JM: JuxtaMembrane; TK; Tyrosine Kinase; SAM: Sterile Alpha Motif. Most vertebrate EphA and B receptors bind promiscuously to any ephrin A or B ligands, respectively (black arrows), with some exceptions, in which the Eph receptor only binds particular ephrin ligand (red arrows). Some class-crossing interactions, in which Eph receptors bind ephrin ligands from a different subtype, also exist (orange arrows). In Drosophila, there is a single Eph receptor and a single Ephrin ligand.
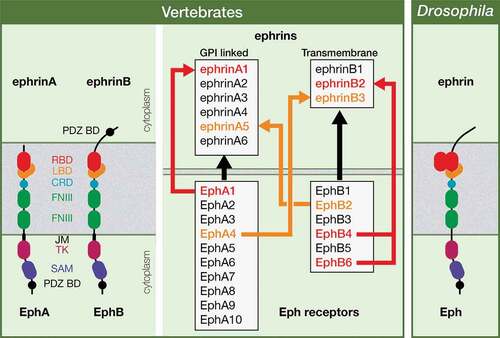
Figure 2. Relevance of mitotic spindle orientation. The orientation of the mitotic spindle is essential as it is going to influence tissue architecture or cell fate specification in the context of a symmetric or an asymmetric cell division, respectively.
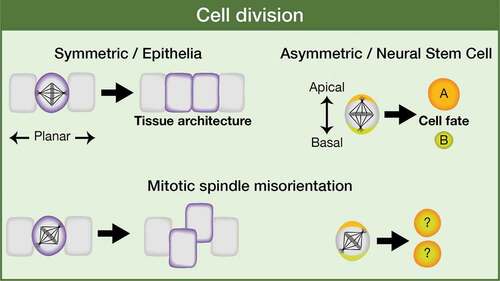
Figure 3. Eph signaling regulates mitotic spindle orientation: working model. Both a reverse and a forward Eph signaling would be operating between neuroepithelial cells in the Drosophila optic lobe. A forward signaling activates Rok/ROCK, which (1) inhibits PI3K-Akt1 signaling pathway and hence proliferation and (2) phosphorylates and activates myosin II Sqh/RLC. A reverse signaling at the level of SA activates aPKC, which fully activate P-Sqh/P-RLC and this, in turn, impacts on spindle orientation by contributing to the correct cortical localization of intrinsic cue regulators, such as Cno/Afadin, Dlg1, and Mud/NuMA. SA: SubApical region; AJs: Adherens Junctions; BL: Basolateral region. (Adapted from Franco and Carmena, 2019).