Figures & data
Figure 1. Effects of melatonin (m) on restraint stress-induced spindle abnormality and chromatin misalignment in MII oocytes. (a) Representative examples of meiotic spindles and chromosomal misalignment in MII oocytes after labeling with α-tubulin antibody (red) and counterstaining of DNA with DAPI (blue) from control mice (Con.), control+M mice (Con.+M), stress mice model (Str.) and stress+M mice (Str.+M). The arrow shows the abnormal spindles (Bar = 10 μm). (b) Incidence of spindle abnormalities and (c) ratio of chromatin misalignment in MII oocytes (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ***P < 0.001 vs. control group; ###P < 0.001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
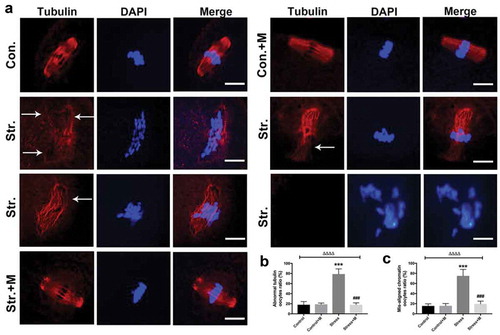
Figure 2. Effects of melatonin (m) and restraint stress on ATP content and mtDNA copy number, expression of ATPIF1 and mitochondria distribution in MII oocytes. (a) ATP content in MII oocytes (n = 50 oocytes from five mice per group). (b) Relative mtDNA copy number in MII oocytes (n = 50 oocytes from five mice per group). (c) ATPIF1 expression, distribution pattern and chromatin alignment in MII oocytes in MII oocytes from control (Con.), control+M (Con.+M), stress (Str.) and stress+M (Str.+M) mice. The arrow shows the chromatin misalignment (Bar = 20 μm). (d) ATPIF1 relative expression (n = 50 oocytes from five mice per group). (e) Abnormal distributed mitochondria oocytes ratio (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ****P < 0.0001 vs. control group; ####P < 0.0001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
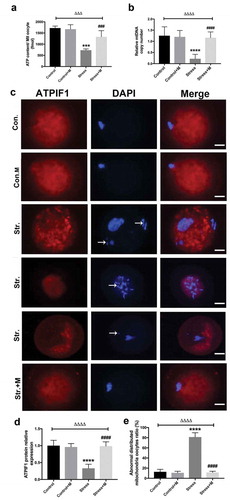
Figure 3. Effects of melatonin (m) and restraint stress on mitochondria membrane potential (ΔΨm). (a) Live oocytes stained with JC-1, where red fluorescence indicates high ΔΨm, and green indicates low ΔΨm from control (Con.), control+M (Con.+M), stress (Str.) and stress+M (Str.+M) mice (Bar = 20 μm). (b) Red to green fluorescence ratio, an indicator of mitochondrial activity (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔP < 0.01 ANOVA; **P < 0.01 vs. control group; ##P < 0.01 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
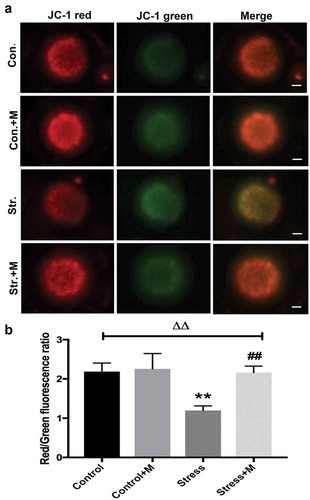
Figure 4. Effects of melatonin (m) and restraint stress on autophagy in MII oocytes. (a) Live oocytes were assessed for autophagic vacuoles, visualized as green fluorescence from control (Con.), control+M (Con.+M), stress (Str.) and stress+M (Str.+M) mice. (Bar = 20 μm). (b) Autophagy levels were quantified as the sum total of green fluorescence within each oocyte (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ****P < 0.0001 vs. control group; ####P < 0.0001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
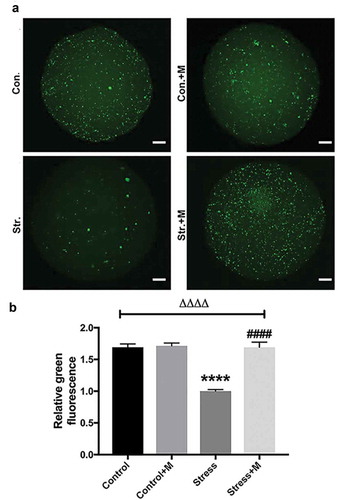
Figure 5. Effects of melatonin (m) and restraint stress on protein level of LC3-II/LC3-I ratio, Atg5, SQSTM1/p62 and SIRT1 in MII oocytes. (a) Western blots and (b-e) the relative expression level of LC3-II/LC3-I ratio, Atg5, SQSTM1/p62 and SIRT1 against ACTB of MII oocytes from per group (50 oocytes per lane, n = 3). ACTB was used as a loading control. All data are presented as mean ± SEM. ΔP < 0.05 ANOVA; ΔΔP < 0.01 ANOVA; ΔΔΔP < 0.001 ANOVA; ΔΔΔΔP < 0.0001 ANOVA; *P < 0.05 vs. control group; **P < 0.01 vs. control group; ***P < 0.001 vs. control group; ##P < 0.01; vs. stress group; ###P < 0.001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
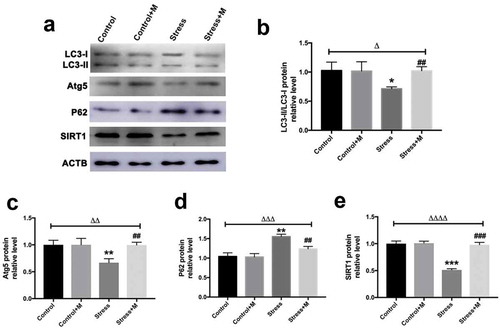
Figure 6. Effects of melatonin (m) and restraint stress on ROS level in MII oocytes. (a) Live oocytes were assessed for ROS, visualized as green fluorescence from control (Con.), control+M (Con.+M), stress (Str.) and stress+M (Str.+D) mice. (Bar = 100 μm). (b) ROS levels were quantified as the sum total of green fluorescence within each oocyte (n = 50 oocytes from five mice per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ****P < 0.0001 vs. control group; ###P < 0.001 vs. stress group. Control, non-stress treated with vehicle; Control+M, non-stress treated with melatonin; Stress, stress treated with vehicle; Stress+M, stress treated with melatonin.
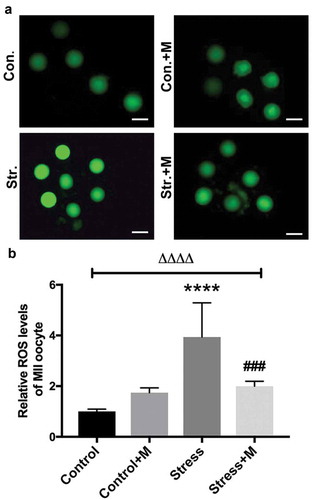
Figure 7. Effects of melatonin and Ex527 treatment on H2O2-induced spindle abnormality and chromatin misalignment as well the protein level of SIRT1 in oocytes in vitro. (a) Micrographs of meiotic spindles and chromosomal misalignment in MII oocytes in control oocytes (Con.), in oocytes treated with melatonin (Con.+M), in oocytes exposed to H2O2 (H2O2), in oocytes treated with H2O2 and melatonin (H2O2 + M) and in oocytes treated with H2O2, melatonin and Ex527 (H2O2 + M+ Ex527) (Bar = 10 μm). (b) Incidence of spindle abnormalities in oocytes of each group (n = 50 oocytes per group). (c-d) Western blots and the relative expression level of SIRT1 against ACTB of oocytes in each group (50 oocytes per lane, n = 3). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ***P < 0.001 vs. control group; ****P < 0.0001 vs. control group; ###P < 0.001 vs. H2O2 group; ####P < 0.0001 vs. H2O2 group. Con., treated with vehicle; Con.+M, treated with melatonin; H2O2, treated with H2O2; H2O2 + M, treated with H2O2 and melatonin; H2O2 + M+ Ex527, treated with H2O2, melatonin and Ex527.
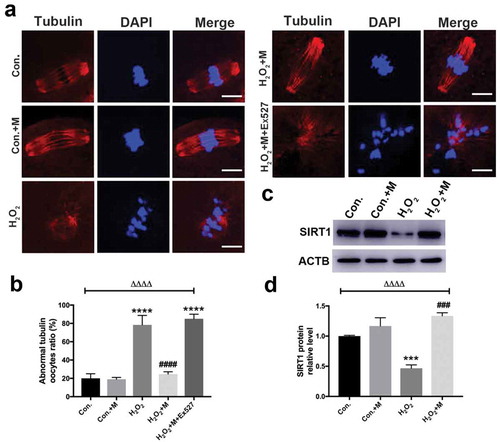
Figure 8. Effects of melatonin and Ex527 treatment on H2O2-induced mitochondrial dysfunction in oocytes in vitro. (a) Live oocytes stained with JC-1, where red fluorescence indicates high ΔΨm, and green indicates low ΔΨm in control oocytes (Con.), in oocytes treated with melatonin (Con.+M), in oocytes exposed to H2O2 (H2O2), in oocytes treated with H2O2 and melatonin (H2O2 + M) and in oocytes treated with H2O2, melatonin and Ex527 (H2O2 + M+ Ex527) (Bar = 20 μm). (b) Red to green fluorescence ratio of JC-1, an indicator of mitochondrial activity (n = 50 oocytes per group). (c) ATP content in oocytes from each group (n = 50 oocytes per group). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; ****P < 0.0001 vs. control group; ####P < 0.0001 vs. H2O2 group. Con., treated with vehicle; Con.+M, treated with melatonin; H2O2, treated with H2O2; H2O2 + M, treated with H2O2 and melatonin; H2O2 + M+ Ex527, treated with H2O2, melatonin and Ex527.
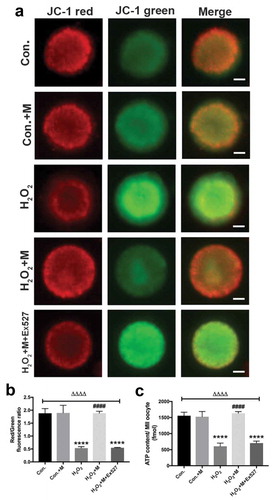
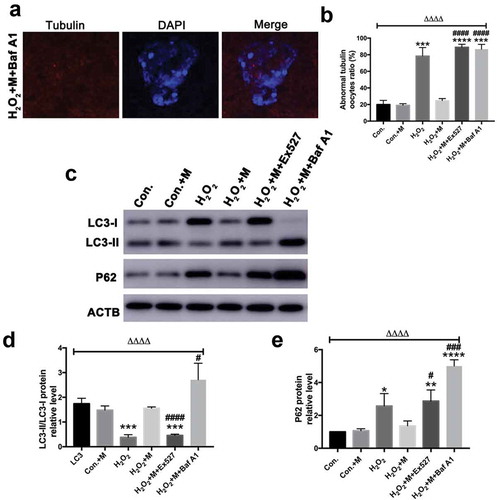
Figure 9. Effects of Baf A1 treatment on H2O2-induced spindle abnormality treated by melatonin and effects of melatonin, Ex527 and Baf A1 treatment on the expression of LC3-II/LC3-I ratio and SQSTM1/p62 in H2O2-treated oocytes. (a) Micrographs of meiotic spindles and chromosomal misalignment in MII oocytes in oocytes treated with H2O2, melatonin and Baf A1 (H2O2 + M+ Baf A1) (Bar = 10 μm). (b) Incidence of spindle abnormalities in oocytes of each group (n = 50 oocytes per group). (c) Western blots and (d-e) the relative expression level of LC3-II/LC3-I ratio and SQSTM1/p62 against ACTB of oocytes in each group (50 oocytes per lane, n = 3). All data are presented as mean ± SEM. ΔΔΔΔP < 0.0001 ANOVA; *P < 0.05 vs. control group; **P < 0.01 vs. control group; ***P < 0.001 vs. control group; ****P < 0.0001 vs. control group; #P < 0.05 vs. H2O2 group; ###P < 0.001 vs. H2O2 group; ####P < 0.0001 vs. H2O2 group. Con., treated with vehicle; Con.+M, treated with melatonin; H2O2, treated with H2O2; H2O2 + M, treated with H2O2 and melatonin; H2O2 + M+ Ex527, treated with H2O2, melatonin and Ex527; H2O2 + M+ Baf A1, treated with H2O2, melatonin and Baf A1.