Figures & data
Table 1. The correlation between TSLNC8 expression and clinicopathological characteristics in lung cancer patients
Figure 1. Expression levels of lncRNA TSLNC8, EGFR and STAT3 in lung cancer tissues. (a). qRT-PCR was used to detect expression of TSLNC8 in clinical lung cancer tissues (n = 31) and normal lung tissues (n = 31). (b). Western blotting was performed to detect expression of EGFR and phosphorylation of STAT3 (Tyr705) in clinical lung cancer tissues (n = 31) and in normal lung tissues (n = 31). GAPDH was used as a loading control. (c). Quantitative analysis of protein levels in B. Data were shown as mean±SD, the result was a representative of three independent experiments. ** p< 0.01 and * p< 0.05
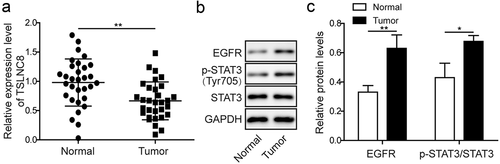
Figure 2. Expression levels of lncRNA TSLNC8, EGFR and STAT3 in lung cancer cell lines. (a). qRT-PCR was used to detect expression of TSLNC8 in human lung cancer cell lines (H358, H460, H1975, H1299, H1395, H1650 and A549) and normal human embryonic lung fibroblast cell line (MRC-5). (b). Western blotting was performed to assess expression of EGFR and phosphorylation of STAT3 (Tyr705) in human lung cancer cell lines and a normal human embryonic lung fibroblast cell line. GAPDH was used as a loading control. (c). Quantitative analysis of protein levels in B. Data were shown as mean±SD, the result was a representative of three independent experiments. *** p< 0.001, ** p< 0.01 and * p< 0.05
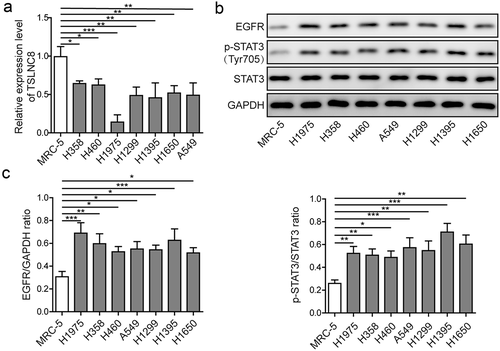
Figure 3. TSLNC8 enhances the effects of osimertinib on cell proliferation, apoptosis, migration and invasion via inhibiting the EGFR-STAT3 pathway. (a). MTT assay was used to evaluate the inhibitory effects of osimertinib on proliferation of H1975 cells. (b). Overexpression of TSLNC8 in the resulting cells was confirmed by qRT-PCR. (c-d). Colony formation assay was performed to evaluate the effects of TSLNC8 overexpression and/or osimertinib administration on proliferation of lung cancer cell line H1975. (e-f). Flow cytometry with Annexin V/PI staining was used to assess the effects of TSLNC8 overexpression and/or osimertinib administration on apoptosis of lung cancer cell line H1975. (g-h). Transwell assay was used to determine the effects of TSLNC8 overexpression and/or osimertinib administration on invasion ability of lung cancer cell line H1975. I-J. In vitro wound healing assay was performed to determine the effects of TSLNC8 overexpression and/or osimertinib administration on migration ability of lung cancer cell line H1975. (k). Western blotting was performed to assess expression of EGFR and phosphorylation of EGFR (Tyr1068) and STAT3 (Tyr705) in lung cancer cell line H1975 in response to TSLNC8 overexpression and/or osimertinib administration. Data were shown as mean±SD, the result was a representative of three independent experiments. *** p< 0.001, ** p< 0.01 and * p< 0.05
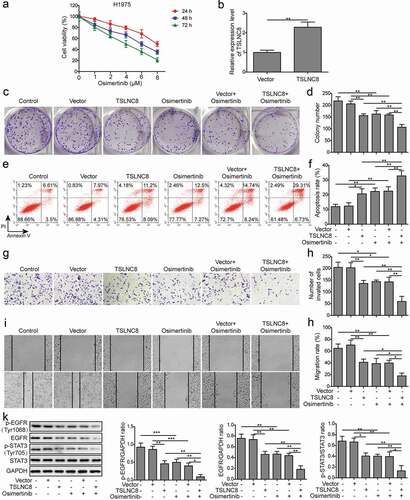
Figure 4. TSLNC8 knockdown suppresses the effects of osimertinib on proliferation, apoptosis, migration and invasion of lung cancer cells. (a). MTT assay was used to evaluate the inhibitory effects of osimertinib on proliferation of H358 cells. (b). Knockdown of TSLNC8 in the resulting cells was confirmed by qRT-PCR. (c-d). Colony formation assay was performed to evaluate the effects of TSLNC8 knockdown and/or osimertinib administration on proliferation of lung cancer cell line H358. (e-f). Flow cytometry with Annexin V/PI staining was used to assess the effects of TSLNC8 knockdown and/or osimertinib administration on apoptosis of lung cancer cell line H358. (g-h). Transwell assay was used to determine the effects of TSLNC8 knockdown and/or osimertinib administration on invasion ability of lung cancer cell line H358. I-J. In vitro wound healing assay was performed to determine the effects of TSLNC8 knockdown and/or osimertinib administration on migration ability of lung cancer cell line H358. Data were shown as mean±SD, the result was a representative of three independent experiments. *** p< 0.001, ** p< 0.01 and * p< 0.05
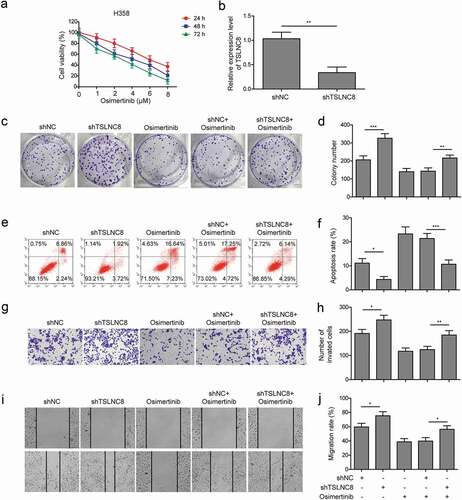
Figure 5. Interaction between TSLNC8 and EGFR and EGFR knockdown inhibits cell proliferation, migration, invasion and promotes apoptosis. (a). Luciferase reporter assay was performed to validate interaction between TSLNC8 and EGFR. (b). Western blotting was performed to assess expression of EGFR and phosphorylation of STAT3 (Tyr705) in lung cancer cell line H1975 in response to EGFR knockdown. GAPDH was used as a loading control. (c). MTT assay was performed to evaluate the effect of EGFR knockdown on proliferation of lung cancer cell line H1975. (d-e). Flow cytometry with Annexin V/PI staining was used to assess the effect of EGFR knockdown on apoptosis of lung cancer cell line H1975. (f-g). Transwell assay was used to determine the effect of EGFR knockdown on invasion ability of lung cancer cell line H1975. (h-i). In vitro wound healing assay was performed to evaluate the effect of EGFR knockdown on migration ability of lung cancer cell line H1975. Data were shown as mean±SD, the result was a representative of three independent experiments. ** p< 0.01 and * p< 0.05
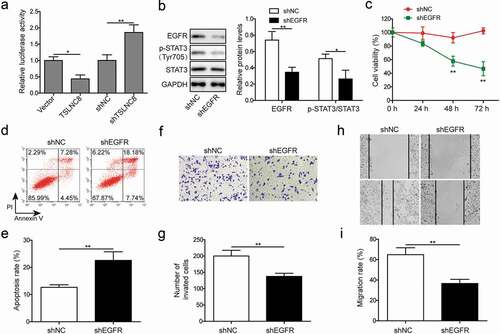
Figure 6. STAT3 inhibition suppresses cell proliferation, apoptosis, migration and invasion. (a). Western blotting was performed to assess expression of EGFR and phosphorylation of STAT3 (Tyr705) in lung cancer cell line H1975 in response to cryptotanshinone treatment. GAPDH was used as a loading control. (b). MTT assay was performed to evaluate the effect of cryptotanshinone on proliferation of lung cancer cell line H1975. (c-d). Flow cytometry with Annexin V/PI staining was used to assess the effect of cryptotanshinone on apoptosis of lung cancer cell line H1975. (e-f). Transwell assay was used to determine the effect of cryptotanshinone on invasion ability of lung cancer cell line H1975. G-H. Wound healing assay was performed to evaluate the effect of cryptotanshinone on migration ability of lung cancer cell line H1975. Data were shown as mean±SD, the result was a representative of three independent experiments. ** p< 0.01 and * p< 0.05
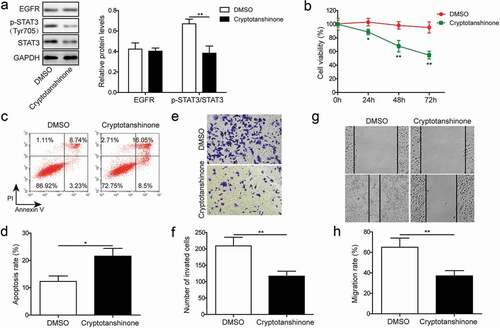
Figure 7. EGFR overexpression and STAT3 signaling activation reverse the effects of TSLNC8 on proliferation, apoptosis, migration and invasion. (a). Western blotting was performed to determine the expression level of EGFR and phosphorylation level of STAT3 (Tyr705) in TSLNC8-overexpressing H1975 cells in response to EGFR overexpression or IL-6 stimulation. GAPDH was used as a loading control. (b). MTT assay was performed to evaluate the effect of EGFR overexpression or IL-6 stimulation on proliferation of H1975 cells that overexpressing TSLNC8. (c-d). Flow cytometry with Annexin V/PI staining was used to assess the influence of EGFR overexpression or IL-6 stimulation on apoptosis of H1975 cells that overexpressing TSLNC8. (e-f). Transwell assay was used to determine the effect of EGFR overexpression or IL-6 stimulation on invasion properties of H1975 cells that overexpressing TSLNC8. (g-h). Wound healing assay was performed to evaluate the effect of EGFR overexpression or IL-6 stimulation on migration ability of H1975 cells that overexpressing TSLNC8. Data were shown as mean±SD, the result was a representative of three independent experiments. ** p< 0.01 and * p< 0.05
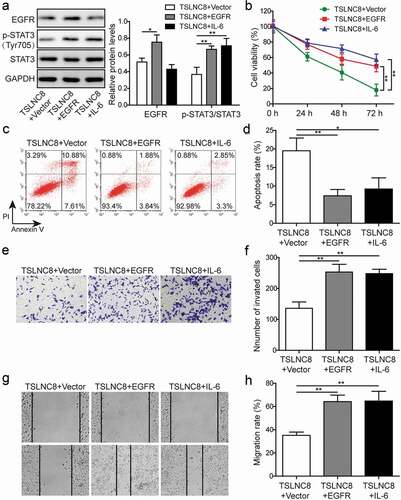
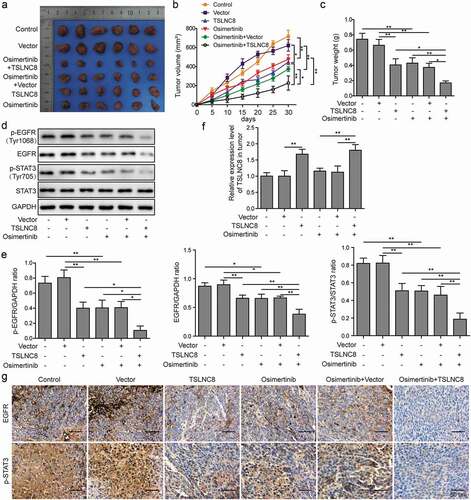
Figure 8. TSLNC8 enhances the inhibitory effects of osimertinib on tumor growth in vivo. (a). Photographs of tumors with TSLNC8 overexpression and/or osimertinib administration. (b). Tumor growth curves of mice model with TSLNC8 overexpression and/or osimertinib administration. Tumor volume (mm3) was measured every 5 days over 4 weeks. (c). Comparison of tumor weights (g) with TSLNC8 overexpression and/or osimertinib administration. (d-e). Western blotting was performed to detect expression of EGFR and phosphorylation of EGFR (Tyr1068) and STAT3 (Tyr705) in tumor tissues in response to TSLNC8 overexpression and/or osimertinib administration. GAPDH was used as a loading control. (f). Overexpression of TSLNC8 by transfection with pWPXL-TSLNC8 was confirmed by qRT-PCR. (g). Immunohistochemistry was performed to detect expression of EGFR and phosphorylation of STAT3 (Tyr705) in tumor tissues in response to TSLNC8 overexpression and/or osimertinib administration. Data were shown as mean±SD, the result was a representative of three independent experiments. ** p< 0.01 and * p< 0.05
Data availability statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
